13
The Effects of Nutrients on Premenstrual Mood Changes
Guy E. Abraham
The premenstrual tension syndromes (PMTS) represent a group of
syndromes occurring premenstrually in a large percentage of women
[1]. The most common PMTS subgroup is PMT-A, characterized by a
premenstrual increase in anxiety, nervous tension, irritability
and mood swings [2]. We will review recent data supporting an
important role of certain nutrients on PMT-A symptomatology and
propose some applications of this knowledge to the nutritional
management of PMT-A.
Effects of Ovarian Steroids on Moods
Estrogens are central nervous system (CNS) stimulants and
progesterone is a CNS depressant [3]. Patients with severe PMT-A
have elevated blood estrogens and/or decreased blood progesterone
during the luteal phase of the menstrual cycle [4]. The low
progesterone/estradiol-17B ratio (P/E2 ratio) is the most
consistent finding.
The mechanism by which estrogens and progesterone influence
moods is not well defined. Estrogens and progesterone influence
monoamine oxydase (MAO) activity. These enzymes are involved in
the oxydation of biogenic amines, such as norepinephrine,
epinephrine, serotonin, dopamine and phenylethylamine. These
biogenic amines affect moods [5-10]. For example, epinephrine
triggers anxiety; norepinephrine, hostility and irritability;
serotonin at high levels creates nervous tension, drowsiness,
palpitation, water retention and inability to concentrate and
perform. Dopamine is believed to balance out the effects of these
three amines by inducing relaxation and increasing mental
alertness [11]. There are two types of MAO: type A, which
deactivates the first four biogenic amines mentioned above, and
type B, which deactivates only dopamine and phenylethylamine
[12]. Estrogens suppress type A and increase type B-MAO
activities, if whereas progesterone increases type A and
suppresses type B-MAO activities [13-16]. Therefore, under
estrogen stimulation, the deactivation of biogenic amines would
be reduced mainly for type-A-MAO-sensitive amines, causing a
relative imbalance, with excess serotonin, norepinephrine and
epinephrine and a relative deficiency of dopamine. This imbalance
would then trigger PMT-A symptoms.
In table I are outlined the dietary precursors of some
neurotransmitters and the effects of these neurotransmitters on
mood and behavior. A very important enzyme in the conversion of
some dietary precursors to the corresponding neurotransmitters is
decarboxylase. The active form of vitamin B-6, pyridoxal
phosphate (PLP), is a required cofactor for the activity of this
enzyme. Under chronic stress, decarboxylase may become the rate
limiting step in the synthesis of these neurotransmitters [17].
Three enzymatic steps are required for the synthesis of
norepinephrine from the amino acid tyrosine: thyrosine
hydroxylase, dopa decarboxylase and dopamine B-hydroxylase (fig.
1). Under chronic stress, tyrosine hydroxylase and
dopamine-B-hydroxylase increase significantly without any change
in dopa decarboxylase, making this latter enzyme rate-limiting.
Such a condition would eventually result in a relative dopamine
deficiency.
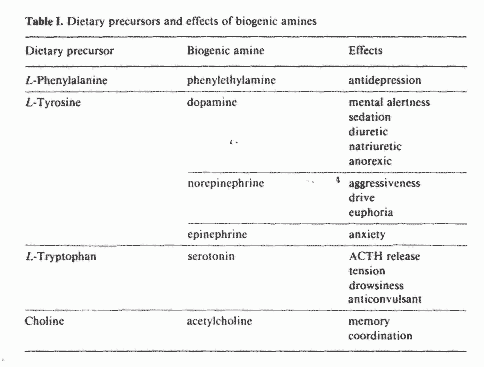
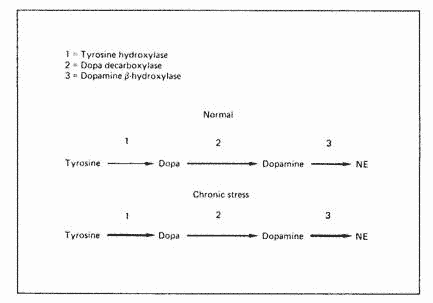
Fig. 1. Effect of chronic stress on the synthesis of CNS
cathecolamines. Reproduced from Abraham [17].
Effect of Certain Nutrients on Biogenic Amines
Independent of estrogen and progesterone effects, magnesium
deficiency may be involved directly in CNS dopamine deficiency.
Although not yet confirmed in humans, studies performed in
laboratory animals revealed that magnesium deficiency causes a
specific depletion of brain dopamine without affecting brain
serotonin and norepinephrine [18].
High intake of refined sugar favors the transfer of tryptophan
from blood to the central nervous system where it is converted to
serotonin [19]. This dietary habit may therefore result in a CNS
serotonin dominance and relative dopamine deficiency.
Effect of Certain Nutrients on Peripheral Levels of Ovarian
Steroids
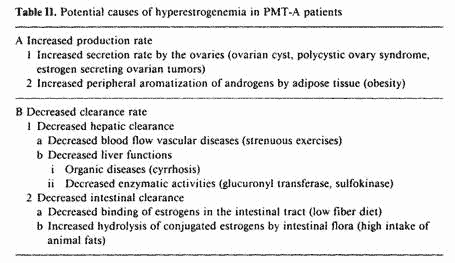
The increased blood E2 levels in PMT-A patients may be due to
increased production rate or decreased clearance rate. Table II
summarizes the factors possibly involved in the hyperestrogenemia
of PMT-A patients. The increased E2 production rate could be the
result of increased secretion rate by the ovary due to ovarian
tumors, functional ovarian cysts and the polycystic ovary
syndrome. PMT-A patients with increased E2 secretion rate do not
respond well to nutritional therapy alone and require either
surgery or ovarian suppression in order to respond to the
nutritional approach.
Decreased clearance rate of estrogens by the liver could be
due to organic lesions such as cyrrhosis. Pyridoxine requires
phosphorylation to become active, and this phosphate transfer
reaction is magnesium dependent. A magnesium deficiency may cause
a relative B-vitamin deficiency. The Biskinds have demonstrated
an important role of the B vitamins in the hepatic metabolism of
estrogens [20-23]. Magnesium deficiency may therefore affect
estrogen metabolism by decreasing the biological activity of the
B vitamins. Vitamin B-6 increases cell membrane transfer and
utilization of magnesium [24]. By increasing utilization of
magnesium, B6 may play a role in the metabolism of estrogens.
Besides its effects on activation of the B vitamins, magnesium
influences estrogens conjugation directly by increasing
glucuronyl transferase activity [25], an enzyme involved in the
hepatic glucuronidation of estrogens.
Another nutritional factor involved in decreased clearance of
estrogens is the role of the intestinal tract in binding and
excreting conjugated estrogens. Whereas food fiber increases
binding and excretion of estrogens [26], animal fats stimulate
the growth of intestinal bacteria, which are capable of
hydrolyzing conjugated estrogens into biologically active free
estrogens [27, 28]. These estrogens are then reabsorbed and
contribute to the hyperestrogenemia of PMT-A patients. A recent
study compared peripheral and fecal estrogens with fiber intake
in 10 vegetarian and 10 omnivorous women who were menstruating
regularly [26]. The omnivorous women consumed 11-13 g of
fiber/day compared to 25-33 g/day for vegetarian women. A
significant negative correlation between fiber intake and blood
estrogens; and positive correlation between fiber intake and
fecal estrogens suggest that food fiber increases the clearance
and fecal excretion of estrogens. Blood estrogen levels were
significantly lower in the vegetarian women than in the
omnivorous subjects. Since women with breast cancer consumed
significantly less fiber than vegetarian controls [28], and women
with severe constipation, due to a lower fiber diet, have a high
prevalence of precancerous lesions of the breast [29], this is of
physiological and clinical significance.
The low peripheral progesterone observed in some PMT-A
patients could be explained by either decreased production rate
and/or increased clearance rate (table III).
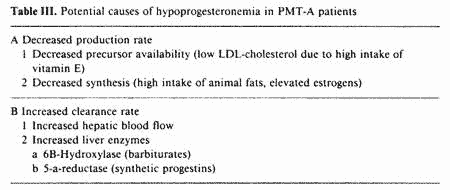
As previously discussed, nutritional deficiencies in B
vitamins and magnesium lower the hepatic clearance rate of
estrogens, with resultant hyperestrogenemia. The luteolytic
effect of elevated estrogens could result in hypoprogesteronemia
[30]. Two other factors possibly involved in decreased
progesterone production rate and independent from estrogen effect
are precursor availability for the synthesis of progesterone and
inhibition of this synthesis by the prostaglandin PGF2a. The
corpus luteum depends almost exclusively on peripheral
cholesterol for steroidogenesis [31].
Low-density-lipoprotein-carried cholesterol (LDL-cholesterol) is
the preferred substrate [31]. Therefore, factors interfering with
this mechanism could affect progesterone synthesis. Data
available from recent studies by London et al. suggest that high
doses of vitamin E may be luteolytic, probably by lowering
LDL-cholesterol ([32] and vide infra).
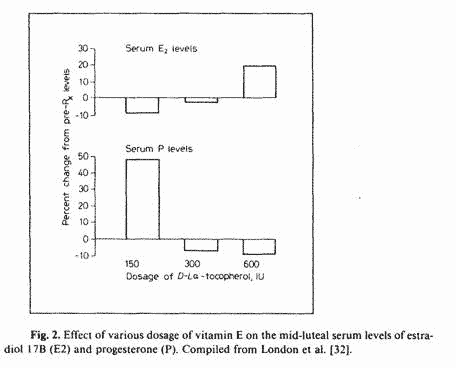
An evaluation of the data available in London’s
publications reveals that although a daily dose of 150 IU vitamin
E raises midluteal serum P levels by 50% (luteotrophic effect),
300 and 600 LU daily were associated with lower P levels than
control levels (fig. 2). Although such decreases in P levels were
not significant, it is likely that dosage of vitamin E greater
than 600 LU will result in greater P suppression. This may be the
explanation for the worsening of PMT-A symptoms with 600 1U of
vitamin E whereas 150 IU showed an improvement of PMT-A symptoms
[33]. The improvement of PMT-D symptoms by the administration of
600 IU of vitamin E [33] is also consistent with this
explanation.
PGF2a is luteolytic in women [34]. The precursor of PGF2a is
arachidonic acid, present in animal fats. Consumption of excess
animal fats increases precursor availability for the synthesis of
PGF2a and therefore may cause luteal deficiency. This could be
the mechanism by which an increased intake of animal fats
predisposes to breast cancer [35], that is, by causing luteal
deficiency, a high risk factor for breast cancer [36]. The
luteotrophic effect of small doses of vitamin F (150 IU or less)
could be due to the inhibitory effect of vitamin Eon the release
of arachidonic acid from storage pool, in this manner decreasing
the availability of PGF2a precursors. The net effect of vitamin F
on P synthesis would depend on the response of LDL-cholesterol
and arachidonate release from storage pools to various dosages of
vitamin E. It is possible that blockage of arachidonate release
is more sensitive to vitamin E than suppression of
LDL-cholesterol, such that low dose of vitamin E would decrease
precursor availability for PGF2a synthesis (a luteotrophic
effect) and high dose of vitamin E would suppress LDL cholesterol
(a luteolytic effect).
Nutritional Management of PMT-A Symptomatology
As previously discussed, moods and behavior are influenced by
biogenic amines present in the CNS. Some biogenic amines are
excitatory and others are inhibitory.
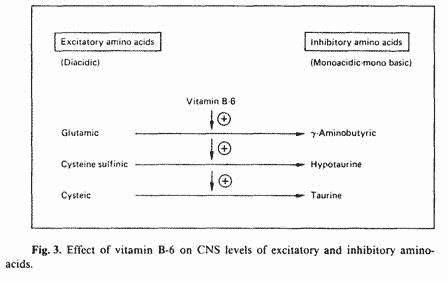
Since PMT-A symptomatology represents an excitatory state of
the CNS; the aim of the nutritional approach is to lower
excitatory biogenic amines and to increase inhibitory biogenic
amines. Vitamin B-6 is unique in its ability to perform this
function. Not only does vitamin B-6 increase CNS levels of the
inhibitory neurotransmitter dopamine, it also increases the
conversion of CNS-active excitatory aminoacids to the
corresponding inhibitory aminoacids. Certain diacidic aminoacids
are CNS excitatory, but upon decarboxylation, the corresponding
monoacidic, monobasic aminoacids have CNS-inhibitory effects
[37]. Vitamin B-6 is a required cofactor in this decarboxylation
reaction (fig. 3). Therefore, the overall effect of vitamin B-6
on the CNS is an increased ratio of inhibitory/excitatory amino
acids. Such an effect would result in sedation. Indeed, we have
reported a significant effect of vitamin B-6 on PMT-A
symptomatology, under double blind conditions [38]. The daily
dosage of 500 mg, however, was in the potentially toxic range.
Recent publications have suggested that vitamin B-6
administration at a daily dose of 2000-6,000 mg results in
peripheral neuropathy [39, 40]. We have also observed that when
vitamin B-6 is given alone without other supplements, some women
tend to develop a B-6 tolerance within 6 months, and require
increased B-6 dosage to produce the same symptomatic relief. We
therefore do not recommend prescribing megadose of vitamin B-6
alone.
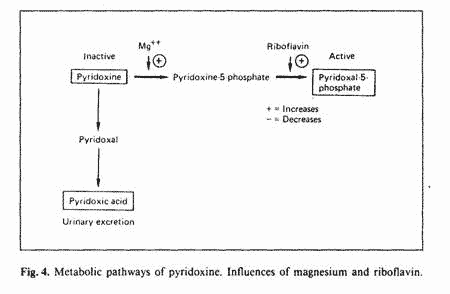
Pyridoxine, the form of vitamin B-6 used in supplement, is
biologically inactive. Two enzymatic steps are required for the
conversion of pyridoxine to pyridoxal phosphate (PLP), the active
form: phosphorilation of pyridoxine, which requires magnesium and
oxydation of pyridoxine phosphate, which requires riboflavin
(fig. 4). The alternate pathway is oxydation of pyridoxine to
pyridoxic acid which is excreted in the urine [43]. This
alternate pathway is used when there is a block in the conversion
of pyridoxine to PLP. If pyridoxine is the toxic form of vitamin
B-6, inefficient metabolism to PLP could be an important factor
in B-6 toxicity. We have recently reported an increased excretion
of pyridoxic acid during the luteal phase of the menstrual cycle
[41], suggesting a block in the conversion of pyridoxine to PLP
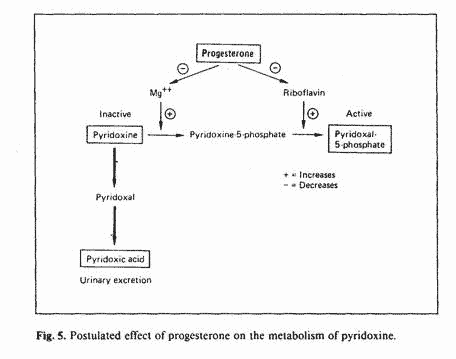
during that phase of the menstrual cycle. Aldosterone levels are
higher during the luteal phase [12]. Aldosterone increases
urinary excretion of magnesium and augments the formation of
flavin nucleotides from riboflavin [43]. Therefore, the
hyperaldosteronism of the luteal phase could explain the block in
the conversion of pyridoxine to PLP, due to depletion of
magnesium and riboflavin. Since progesterone increases
aldosterone secretion [44, 45] progesterone administration could
predispose to vitamin B-6 neurotoxicity (fig. 5).
To minimize side effects of vitamin B-6 therapy, other
micronutrients should be given together with vitamin B-6, mainly
magnesium and the B-vitamins. Based on available data in the
literature regarding dietary status of PMTS patients, an OTC
nutritional supplement (Optivite) was formulated for use in the
nutritional management of PMTS [30, 46]. Under double blind
conditions, this supplement significantly decreased PMT-A
symptomatology compared to placebo, when given at a daily dose of
6 tablets containing 300 mg of vitamin B-6 [47]. Decreased serum
estradiol-17B and increased serum progesterone levels were
observed during the midiluteal phase of PMTS patients following
3-6 months of Optivite administration at daily dosage containing
300-600 mg of pyridoxine [48].There has been so far no reported
case of peripheral neuropathy following this supplement, even
though it has been prescribed by physicians nationwide for PMTS
patients over the past 6 years, at daily doses tablets which
contain 100-600 mg pyridoxine.
In terms of macronutrients, the following recommendations are
in order in PMT-A patients: (1) Limit intake of simple
carbohydrates to 15% of calories because they increase CNS
serotonin, a biogenic amine involved in nervous tension [49]. (2)
Caffeine-containing foods and drinks should be curtailed since
caffeine is a CNS stimulant. (3) Limit intake of dairy products
to 2 servings a day, because they block magnesium absorption and
increase urinary excretion of magnesium [50]. (4) Limit total
fats to 30% of calories, with twice as much vegetable as animal
fats. As we have discussed above, animal fats cause
hyperestrogenemia, and suppresses ovarian secretion of
progesterone. (5) Limit daily intake of vegetable proteins to 1
g/kg body weight, and animal proteins to 0.5 g/kg body weight.
Preliminary data suggests that a vegetable/animal ratio of
protein consumed equal to or greater than two decreases the
prevalence of PMTS symptomatology [50]. (6) Increase dietary
fiber intake to 20-40 g a day. Food fiber helps the bowel
eliminate estrogens [26].
The PMTS problem presents a unique opportunity to study the
relationship between nutrients and brain functions. This is a
great challenge for multidisciplinary research with tremendous
clinical applications.
References
1 Hargrove, J.T.; Abraham, G.E.: The incidence of premenstrual
tension in a gynecologic clinic. J. reprod. Med. 27: 721-724
(1982).
2 Abraham, G.E.: Premenstrual tension. Curr. Prob. obstet.
Gynec. 3: 5 (1980).
3 Abraham, G.E.: Nutrition and the premenstrual tension
syndromes. J. appl. Nutr. 36: 103-124 (1984).
4 Mertz, W.: Human requirements. Basic and optimal. Ann. N.Y.
Acad. Sci. 199: 191 (1972).
5 Coppen, A.; Shaw, D.M.: Potentiation of the antidepressive
effect of a monoamine oxidase inhibitor by tryptophan. Lancet i:
79 (1963).
6 Geller, E.; Ritvo, E.R.; Freeman, B.J.; et al.: Preliminary
observations on the effect of fenfluramine on blood serotonin and
symptoms in three autistic boys. New Engl. J. Med. 307: 165
(1982).
7 Hollander, W.; Michelson, A.L.; Wilkins, R.W.: Serotonin and
antiserotonins. Circulation 26: 246 (1957).
8 Pollin, W.; Cardon, P.V.; Kety, S.S.: Effects of amino acid
feedings in schizophrenic patients treated with Iproniazid.
Science 133: 104 (1961).
9 Schilldkraut, J.J.; Kety, S.S.: Biogenic amines and
emotions. Science 152: 21 (1967).
10 Smith, B.; Prockop, D.J.: Central-nervous-system effects of
ingestion of L-tryptophan by normal subjects. New Engl. J. Med.
267: 1338 (1962).
11 Boshes, B.; Arbit, J.: A controlled study of the effect of
L-dopa upon selected cognitive and behavioral functions. Trans.
Am. neurol. Ass. 55: 59 (1970).
12 Tipton, K.F.; Houslay, M.D.; Mantle, T.J.: The nature and
locations of the multiple forms of monoamine oxidase; in Kety,
Monoamine oxidase and its inhibition. Ciba Foundation Symposium
39, pp. 5-16 (Elsevier-North Holland, New York 1976).
13 Belmaker, R.H.; Murphy, D.L.; Wyatt, R.J.; et al.: Human
platelet monoamine oxidase changes during the menstrual cycle.
Archs gen. Psychiat. 31: 553 (1974).
14 Briggs, M.: Relationship between monoamine oxidase activity
and sex hormone concentration in human blood plasma. J. Reprod.
Fertil. 29: 447 (1972).
15 Klaiber, E.L.; Kobayashi, Y.; Broverman, D.M.; et al.:
Plasma monoamine oxidase activity in regularly menstruating women
and in amenorrehic women receiving cyclic treatment with
estrogens and a progestin. J. clin. Endocr. 33: 630 (1971).
16 Redmond, D.E.; Murphy, D.L.; Baulu, J.; et al.: Menstrual
cycle and ovarian hormone effects on plasma and platelet
monoamine oxidase (MAO) and plasma dopamine-B-hydroxylase (DBH)
activities in the rhesus monkey. Psychosom. Med. 37:
417(1975).
17 Abraham, G.E.: The premenstrual tension syndromes. Contr.
Obstet. Gynec. Nurs. 3: 170(1980).
18 Barbeau, A.; Rojo-Ortea, J.M.; Brecht, H.M.; et al.:
Deficience en magnesium et dopamine cérébrale;
First Int. Symp. Magnesium Deficit in Human Pathology, Paris
1973.
19 Wurtman, R.J.: Control of neurotransmitter synthesis by
precursor availability and food consumption; in Naftolin, Ryan,
Davies, Subcellular mechanisms in reproductive
neuroendocrinology, pp. 149-166 (Elsevier, New York 1975).
20 Biskind, M.S.: Nutritional deficiency in the etiology of
menorrhagia, cystic mastitis and premenstrual tension. Treatment
with vitamin B complex. J. clin. Endocr. Metab. 3: 227-234
(1943).
21 Biskind, M.S.; Biskind, G.R.: Effect of vitamin B complex
deficiency on inactivation of estrone in the liver. Endocrinology
31: 109-114 (1942).
22 Biskind, M.S.; Biskind, G.R.: Inactivation of testosterone
propionate in the liver during vitamin B complex deficiency.
Alteration of the estrogen-androgen equilibrium. Endocrinology
32: 97-102 (1945).
23 Biskind, MS.; Biskind, GR.: Biskind, L.H.: Nutritional
deficiency in the etiology of menorrhagia, metrorrhagia, cystic
mastitis, and premenstrual tension. Surgery Gynec. Obstet. 78:
49-57 (1944).
24 Abraham, G.E.; Schwartz, U.D.; Libran, M.M.: Effect of
vitamin B-6 on plasma and red blood cell magnesium levels in
premenopausal women. Ann. clin. Lab. Sci. 11: 333 (1981).
25 Brown, R.C.; Bidlack, W.R.: Regulation of glucuronlyl
transferase by intracellular magnesium. Proc. Int. Symp.
Magnesium and Its Relationship to Cardiovascular, Renal and
Metabolic Disorders, Los Angeles 1985.
26 Goldin, B.R.; et al.: Estrogen excretion patterns and
plasma levels in vegetarian and omnivorous women. New Engl. J.
Med. 307: 1542-1547 (1982).
27 Goldin, B.R.; Gorbach, S.L.: The relationship between diet
and rat fecal bacterial enzymes implicated in colon cancer. J.
natn. Cancer Inst. 57: 371 (1976).
28 Adlercreutz, H.; Fotsis, T.; Heikkinen, R.; et al.:
Excretion of the lignans enterolactone and enterodiol and of
equol in omnivorous and vegetarian postmenopausal women and in
women with breast cancer. Lancet ii: 1295 (1982).
29 Petrakis, N.L.; King, F.B.: Cytological abnormalities in
nipple aspirates of breast fluid in women with severe
constipation. Lancet ii: 1203-1205 (1981).
30 Abraham, G.E.: Nutritional factors in the etiology of the
premenstrual tension syndromes. J. reprod. Med. 28: 446-464
(1983).
31 Gwynne, J.T.; Strauss, J.F., III: The role of lipoproteins
in steroidogenesis and cholesterol metabolism in steroidogenic
glands. Endocr. Rev. 3: 299 (1982).
32 London, R.S.; Sundaram, G.; Manimekalai, S.; et al.: The
effect of alpha-tocopherol on premenstrual symptomatology. A
double-blind study. H. Endocrin correlates. J. Am. Coll. Nutr. 3:
351 (1984).
33 London, R.S.; Sundaram, G.S.; Murphy, L.; et al.: The
effect of alpha-tocopherol on premenstrual symptomatology. A
double-blind study. J. Am. Coil. Nutr. 2: 115 (1983).
34 Dennefors, B.L.; Sjogren, A.; Hamberger, L.: Progesterone
and adenosine 3’, 5’- monophosphate formation by
isolated human corpora lutea of different ages. Influence of
human chronic gonadotropin and prostaglandins. J. clin. Endocr.
Metab. 55: 102
(1982).
35 Wynder, E.L.; Cohen, L.A.; Hill, P.: Nutrition and the
etiology and prevention of breast cancer; in Strax, Control of
breast cancer through mass screening, pp. 89-100 (Littleton,
Colorado 1979).
36 Cowan, L.D.; Geordies, L.; Tunisia, J.A.; et al.: Breast
cancer incidence in women with a history of progesterone
deficiency. Am. J. Epidem. 114: 209 (1981).
37 Huxtable, R.J.: Tissue taurine concentrations; in Huxtable,
Advances in experimental medicine and biology, Peasants-Morales,
pp. 401-426 (Plenum Press, New York 1982).
38 Abraham, G.E.; Hargrove, J.T.: Effect of vitamin B6 on
premenstrual symptomatology in women with premenstrual tension
syndromes. A double blind crossover study. Infertility 3: 155
(1980).
39 Schaumburg, H.; Kaplan, J.; Windebank, A.: Sensory
neuropathy from pyridoxine abuse. New Engl. J. Med. 309: 445
(1983).
40 Parry, G.J.; Broaden, D.E.: Sensory neuropathy with low
dose pyridoxine. Neurology, Minneap. 35: 1466-1468 (1985).
41 Abraham, G.E.: Bioavailability of selected nutrients from a
dietary supplement. Optivite for women. J. appl. Nutr. 37: 67
(1985).
42 Abraham, G.E.: The normal menstrual cycle; in Givens,
Endocrine causes of menstrual disorders, pp. 15-44 (Year Book
Medical Publishers, Chicago 1978).
43 Oelkers, W.; Schoneshofer, M.; Blumel, A.: Effects of
progesterone and four synthetic progestagens on sodium balance
and the renin-aldosterone system in man. J. clin. Endocr. Metab.
39: 882 (1974).
44 Sundsfjord, J.: Plasma renin activity and aldosterone
excretion during prolonged progesterone administration. Acta
endocr. 67: 483 (1971).
45 Goei, G.S.; Abraham, G.E.: Effect of nutritional
supplement, optivite on premenstrual symptomatology in patients
with premenstrual tension. J. reprod. Med. 28: 527 (1983).
46 Chakmakjian, Z.H.; Higgins, C.E.; Abraham, G.E.: the effect
of a nutritional supplement, optivite for women, on premenstrual
tension syndromes. Effect on symptomatology, using a double-blind
cross-over design. J. appl. Nutr. 37: 12 (1985).
47 Fuchs, N.; Hakim, M.; Abraham, G.E.: The effect of a
nutritional supplement, optivite for women, on premenstrual
tension syndromes. Effect on blood chemistry and serum steroid
levels during the midluteal phase. J. appl. Nutr. 37:
1(1985).
48 Wurtman, R.J.: Control of neurotransmitter synthesis by
precursor availability and food consumption; in Naftolin, Ryan,
Davies, Subcellular mechanisms in reproductive
neuroendocrinology, pp. 149-166 (Elsevier, New York 1975).
49 Abraham, G.E.: Nutrition and the premenstrual tension
syndromes. J. appl. Nutr. 36: 103-124 (1984).
Guy E. Abraham, MD, Optimox, Inc., 2720 Monterey Street No.
406, Torrance, CA 90503 (USA)
This page was first uploaded to The Magnesium Web Site on July
19, 2002
http://www.mgwater.com/








