Reprinted from THE JOURNAL OF REPRODUCTIVE MEDICINE Vol. 35
No. 5, May 1990
A Total Dietary Program Emphasizing Magnesium Instead of
Calcium
Effect on the Mineral Density of Calcaneous Bone in
Postmenopausal Women on Hormonal Therapy
Guy E. Abraham, M.D.
Harinder Grewal, M.D.
The use of calcium supplementation for the
management of primary postmenopausal osteoporosis (PPMO) has
increased significantly in the past few years. A review of the
published data does not support calcium megadosing during
postmenopause. Controlled studies showed no significant effect of
calcium intake on mineral density of trabecular bone and a slight
effect on cortical bone. Since PPMO is predominantly due to
demineralization of trabecular bone, there is no justification
for calcium mega dosing in postmenopausal women. Soft tissue
calcification is a serious risk factor during calcium megadosing
under certain conditions. A total dietary program emphasizing
magnesium instead of calcium for the management of PPMO takes
into account the available data on the effects of magnesium,
life-style and dietary habits on bone integrity and PPMO. When
this dietary program was tested on 19 postmenopausal women on
hormonal replacement therapy who were compared to 7 control
postmenopausal women, a significant increase in mineral bone
density of the calcaneous bone (BMD) was observed within one
year. Fifteen of the 19 women had had BMD below the spine
fracture threshold before treatment; within one year, only 7 of
them still had BMD values below that threshold.
Introduction
Osteoporosis is a clinical syndrome characterized by a high
susceptibility to bone fracture with resultant symptomatology and
is due to an excessive amount of bone loss without any change in
bone structure.1, 2 It is called “primary
osteoporosis” when there are no known factors and includes
senile osteoporosis, with both cortical and trabecular bone loss,
and postmenopausal osteoporosis (PPMO), with mainly trabecular
bone loss during the first decade after menopause.3,
4
During the last National Institutes of Health work shop on
osteoporosis, in 1987, the panel of experts recommended a daily
intake of 1,500mg of calcium by postmenopausal women as a means
of preventing PPMO even though the clinical studies presented in
the same proceedings reached different conclusions 6,
7: calcium supplementation in daily amounts of less than or
equal to 3,000 mg had no significant effect on postmenopausal
trabecular bone loss above the placebo effect.
One of us (G.E.A.) previously postulated that PPMO is a
skeletal manifestation of magnesium deficiency with the corollary
that postmenopausal trabecular bone loss would not occur even
without estrogen therapy if the magnesium intake were sufficient
to maintain an adequate bone magnesium reserve. That author
proposed a total dietary program emphasizing magnesium instead of
calcium, not unlike the dietary recommendations for women with
the premenstrual tension syndrome.
To test the above postulate, the magnesium- emphasized program
was implemented for 6-12 months in 19 postmenopausal women
receiving hormonal replacement therapy. Seven postmenopausal
women on hormonal replacement therapy served as controls.
Trabecular bone density was assessed with single photon
densitometry of the calcaneous bone.
Materials and Methods
Twenty-six postmenopausal women were recruited from a
menopause clinic. All subjects were on hormonal replacement
therapy, either estrogen alone in those with surgical menopause
or cyclic progestogen superimposed on estrogen therapy in those
with an intact uterus.
All patients underwent bone density measurement of the
calcaneous bone (BMD) with single photon absorptiometry as
described by Ross et al and were advised about the dietary
program (Figure 1). Micronutrients were supplied in the form of a
complete multivitamin, multimineral supplement (Gynovite,
Optimox, Inc., Torrance, CA) containing 500 mg of calcium as the
citrate salt and 200 mg of magnesium as the oxide (Table 1).
Seven patients received dietary advice but chose not to take the
supplement. Nineteen patients received dietary advice and
ingested six tablets of the nutritional supplement daily. Besides
the supplement, magnesium oxide was given at a daily dosage of
400 mg of elemental magnesium. Therefore, the 19 patients
received 50% of the recommended daily allowance (RDA) of calcium
and 200% of the RDA of magnesium for women. In all 26 patients,
BMD of the calcaneous bone was repeated at their return visit,
6-12 months later. The radioactive materials were replaced every
three months to prevent artifacts from significant radioactive
decay, resulting in a prolonged counting time during BMD
measurement.


Results
Comparing the two groups of patients, there was no significant
difference in age, height, weight, years since menopause,
duration of hormone therapy, base line BMD or duration of
follow-up (Table II).
A nonsignificant increase, 0.7%, in the mean BMD of the seven
patients receiving hormonal therapy and dietary advice was
observed as compared to a mean increase of 11% in the 19 women
receiving the supplements (P < .01 by paired data analysis)
(Table II).
Discussion
Since several nutrients besides calcium are important for bone
integrity,11-24 a total dietary program would be
preferable to calcium supplementation in the prevention and
management of PPMO.
Albanese et al 11 reported that calcium
supplementation alone was two to three times less effective on
phalanx bone density in women aged 38-66 years than was
nutritional supplementation containing the same amount of calcium
plus some essential trace elements and vitamins at RDA levels.
However, Albanese’s supplement lacked several essential
micronutrients and did not contain magnesium.
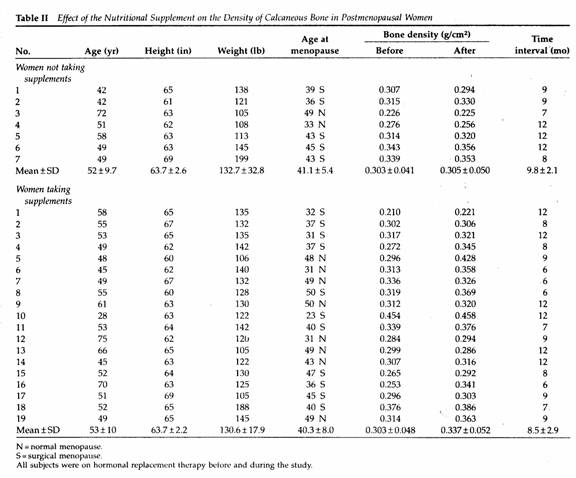
The results of the present study suggest that complete
nutritional supplementation containing 500 mg of calcium as the
citrate salt and 600 mg of magnesium as the oxide has a
significant effect on reversing postmenopausal bone loss of the
calcaneous bone within a relatively short period of time in
patients receiving hormonal replacement therapy for several
years. The effect of this magnesium-emphasized program on
calcaneous bone density was 16 times greater than that of dietary
advice alone in postmenopausal women on hormonal replacement
therapy. Ross et al 10 defines the spine fracture
threshold as a BMD of 0.32 g/cm of the calcaneous bone. In 15 of
the 19 women receiving the supplement the BMD was below the
fracture threshold before treatment. Within a year after the
program only seven patients had BMD values <0.32 g/cm (Table
II).
Dalderup was the first to report, in 1960, a possible role of
magnesium in therapy for osteoporosis He also warned against soft
tissue calcification caused by calcium and vitamin D
megadosing.
A recent literature review suggested that the magnesium
content of the food supply in Europe and North America results in
a daily intake averaging 72-161 mg below women’s RDA of 300
mg. Magnesium supplementation ranging from 67 to 600 mg daily
improved several clinical problems in those countries.
The RDA of magnesium for Soviet women varies from 500 to 1250
mg, depending on physiologic conditions. It is unlikely that
genetic factors account for such a difference in magnesium
requirements between Soviet and U.S. women. The U.S. RDA of
magnesium, based on short-term balance studies, probably is the
minimum daily intake of magnesium that the human body can adjust
to but at the cost of increased susceptibility to stress and,
very probably, PPMO.8 Long-term balance studies have
indicated much greater needs for magnesium than the U.S. RDA, and
>1,000 mg/d is sometimes required to maintain a positive
balance under stressful conditions.
It was postulated by one of us (G.EA.) that PPMO is
predominantly a skeletal manifestation of chronic magnesium
deficiency, facilitated by estrogen withdrawal during
postmenopause. If this postulate is correct and properly designed
clinical trials could test it easily, postmenopausal trabecular
bone loss might not occur even without estrogen replacement
therapy as long as the magnesium intake and bone magnesium
reserve were adequate. Even senile osteoporosis would be
prevented by this program because the high-magnesium diet would
lower the calcium threshold. Raising the RDA of magnesium to
1,000 mg/d and lowering that for calcium to 500 mg/d might be the
most cost-effective approach to PPMO at a national level. This
proposed RDA for calcium would be more in line with the World
Health Organization’s “practical allowance” of
400-500mg daily for adults. Such a reversal of the
magnesium:calcium ratio in the RDA recommendation most probably
would lower the incidence and prevalence of many other
degenerative diseases and pregnancy complications caused in part
by magnesium deficiency.
We are now undertaking a more comprehensive study of the
magnesium-emphasized dietary program in postmenopausal women not
receiving hormonal replacement because of contraindications to
estrogen use. The daily supplementation of elemental magnesium
will vary from 200 to 1,000 mg. If the above postulate is valid,
BMD changes would be expected to correlate positively with the
amount of magnesium ingested.
If, indeed, a magnesium-emphasized dietary program reverses
bone loss in PPMO, this program for PPMO might be the most
cost-effective one and essentially devoid of side effects.
References
1. National Institutes of Health Consensus Development
Conference: Osteoporosis. JAMA 252:799, 1984
2. National Institutes of Health Consensus Development
Conference on Osteoporosis, Bethesda, April 1984
3. Riggs BL, Melton LJ III: Heterogeneity of involutional
osteoporosis: Evidence for two osteoporosis syndromes. Am J Med
75:899, 1983
4. Johnston CC, Norton J, Khairi MRA, et al: Heterogeneity of
fracture syndromes in postmenopausal women. J Clin Endocrinol
Metab 61:551, 1985
5. Chesnut CH: Report from the NIH Consensus Conference, 1984,
and NIH/NOF Workshop, 1987. in Osteoporosis Update, 1987. Edited
by HK Genant. San Francisco, Radiology Research and Education
Foundation, 1987, pp 3-6
6. Ettinger B: Estrogen, progestogen, and calcium in treatment
of postmenopausal women In Osteoporosis Update, 1987. Edited by
HK Genant. San Francisco, Radiology Research and Education
Foundation, 1987, pp 253-58
7. Christiansen C, Riis BJ: Optimal prophylaxis for
postmenopausal bone loss. in Osteoporosis Update, 1987. Edited by
HK Genant. San Francisco, Radiology Research Education
Foundation, 1987, pp 259-266
8. Abraham GE: The calcium controversy. J Appl Nutr 34:69,
1982
9. Abraham GE: Nutritional factors in the etiology of the pre
menstrual tension syndromes. J Reprod Med 28:446, 1983
10. Ross PD,Wasnich RD, Heilbrun LK, et al: Definition of a
spine fracture threshold based upon prospective fracture risk.
Bone 8:271, 1987
11. Albanese AA, Lorenze EJ, Edelson HA, et al: Calcium
nutrition and skeletal bone health. Nutr Rep Int 38:211, 1988
12. Bariscoe AM, Ragen C: Relation of magnesium on calcium
metabolism in man. Am J Clin Nutr 19:296, 1966
13. Benke PJ, Fleshood HL, Pitot HC: Osteoporotic bone disease
in the pyridoxine-deficient rat. Biochemical Med 6:526, 1972
14. Bikle DD, Murphy EW, Rasmussen H: The ionic control 1.25-
dihydroxyvitamin D synthesis in isolated chick renal
mitochondria: The role of potassium. Biochem Biophys Acta
437:394, 1976
15. Carlisle EM: Silicon as an essential trace element in
animal nutrition. in Silicon Biochemistry. Edited by ED
O’Connor. New York, John Wiley & Sons, 1986, pp
123-39
16. Cohen L, Kitzes R: Infrared spectroscopy and magnesium
content of bone mineral in osteoporotic women. Isr J Med Sci
17:1123, 1981
17. Freudenheim JL, Johnson NE, Smith EL: Relationships
between usual nutrient intake and bone-mineral content of women
35-65 years of age: Longitudinal and cross-sectional analysis. Am
J Clin Nutr 44:863, 1986V
18. Medalle R, Waterhouse C, Hahn TJ: Vitamin D resistance in
magnesium deficiency. Am J Clin Nutr 29:858, 1976
19. Mirra JM, Alcock NW, Shils ME: Effects of calcium and
magnesium deficiencies on rat skeletal development and
parathyroid gland area. Magnesium 1:16, 1982
20. Nielsen FH, Hunt CD, Mullen LM: Effect of boron on
mineral, estrogen, and testosterone metabolism in postmenopausal
women. Fed Am Soc Exp Biol J 1:394, 1987
21. Okazaki M: Magnesium action on the stability of
fluorapatite. Magnesium 7:148, 1988
22. Okazaki M: MG during hydroxyapatite formation. Magnesium
6:296, 1987
23. Strause LG, Hegenauer J, Saltman P: Effects of long-term
dietary manganese and copper deficiency on rat skeleton. Nutr
116:135, 1986
24. Yano K, Heilbrun LK, Wasnich RD, et al: The relationship
between diet and bone mineral content of multiple skeletal sites
in elderly Japanese-American men and women living in Hawaii. Am J
Clin Nutr 42:877, 1985
25. Dalderup LM: The role of magnesium in osteoporosis and
idiopathic hypercalcaemia. Voeding 21:424, 1960
26. Marier JR: Magnesium content of the food supply in the
modern-day world. Magnesium 5:1, 1986
27. Lederer J: Magnesium: Mythes et réalité.
Paris, Maloine Editeurs, 1984, p 54
28. Seelig MS: Magnesium requirements in human nutrition.
Magnesium Bull 1a:26, 1981
29. Heroux O, Peter D, Heggteveit HA: Long-term effect of
suboptimal dietary magnesium. J Nutr 107:1640, 1977
30. Kanis JA, Passmore R: Calcium supplementation of the diet:
I and II. Br Med J 298:137, 205, 1989
31. Seelig MS: Magnesium Deficiency in the Pathogenesis of
Disease. New York, Plenum Medical Book Company, 1980
From Optimox, Inc., Torrance, and Women’s Life Care,
Anaheim Hills, California.
Address reprint requests to: Guy E. Abraham, M.D., 2720
Monterey Street, Suite 406, Torrance, CA 90503.
This page was first uploaded to The Magnesium Web Site on July
20, 2002
http://www.mgwater.com/



