In American Journal of Cardiology 63:4G-21G, 1989
CARDIOVASCULAR CONSEQUENCES OF MAGNESIUM DEFICIENCY AND LOSS:
PATHOGENESIS, PREVALENCE AND MANIFESTATIONS. MAGNESIUM AND
CHLORIDE LOSS IN REFRACTORY POTASSIUM REPLETION
Mildred S. Seelig, M.D., M.P.H.
New York Medical College, Valhalla, and the American College
of Nutrition, Scarsdale, New York
The section headers of this paper are as follows:
Dietary magnesium (Mg) deficiency is more prevalent than
generally suspected, and can cause cardiovascular lesions leading
to disease at all stages of life. The average American diets is
deficient in Mg, especially in the young, in alcoholic persons,
and in those under stress or with diseases or receiving certain
drug therapies, who have increased Mg needs. Otherwise normal, Mg
deficient diets cause arterial and myocardial lesions in all
animals, and diets that are atherogenic, thrombogenic and
cardiovasopathic, as well as Mg-deficient, intensify the
cardiovascular lesions, whereas Mg supplementation prevents them.
Diuretics and digitalis can intensify an underlying Mg
deficiency, leading to cardiac arrhythmias that are refractory
unless Mg is added to the regimen. Potassium (K) depletion in
diuretic-treated hypertensive has been linked to an increased
incidence of ventricular ectopy and sudden death. K
supplementation alone is not the answer. Mg has been found to be
necessary to intracellular K repletion in these patients. Because
patients with congestive heart failure and others receiving
diuretic therapy are also prone to chloride loss, leading to
metabolic alkalosis that also interferes with K repletion, the
addition of Mg and Cl supplements in addition to the K seems
prudent.
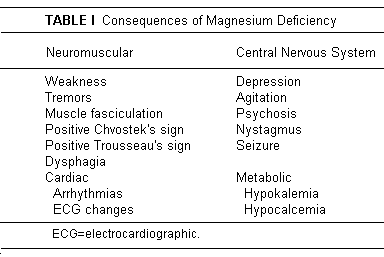
It is paradoxical that many physicians do not consider
magnesium (Mg) a nutrient, inadequacy of which has many overt
consequences (Table I),
precisely because it has long been recognized as a very
effective drug for the treatment of convulsions and hypertension
of eclampsia,(1-3), and for cardiac arrhythmias, including those
that are refractory without Mg therapy.(1,4-9) Realization that
its efficacy in these life-threatening conditions might be a
reflection of repair of its deficiency, as well as of its
pharmacologic activity, has been gradual. Basic studies have
clarified mechanisms by which Mg deficiency can disrupt arterial
and cardiac integrity; Mg plays many roles at the mitochondrial
level, activating numerous enzymes and preserving both function
and structure. (10,11) The heart, with its dense mitochondrial
structure and high enzymatic activity,(12) is particularly
vulnerable to Mg loss. There is substantial evidence that
long-term Mg deficiency in animal species, including monkeys,
causes cardiovascular histologic and functional
abnormalities.(13) Whereas Mg deficiency in all experimental
models increases vulnerability to cardiotoxic agents, and
intensifies arterial and cardiac damage caused by other dietary
factors (such as excesses of fat, calcemic agents and inorganic
phosphate) Mg supplementation has a protective effect.(1,13,14)
The epidemiologic evidence that populations consuming more Mg
from hard water or diet are less prone to cardiovascular disease
and sudden death, than those with lower Mg intakes,(15-19)
substantiates the relevance of the animal findings to human
atherosclerosis, hypertension and its treatment, myocardial
infarction, and sudden cardiac death.(1,20) Both Mg loss and
hypochloremic alkalosis interfere with potassium (K)
repletion(21-24) and contribute to the risk of sudden death among
hypertensive and cardiac patients treated with Mg- and K-losing
diuretics.(25)
It is widely assumed, especially in the United States where
the food intake is generally ample, that Mg inadequacy is
unlikely. However, the foods that are highest in Mg - vegetables
(especially legumes and dark green leafy vegetables), fish
(including shellfish, and whole grains and nuts (Table
II(26))
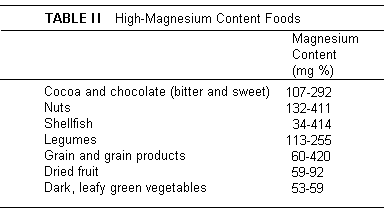
are not major constituents of the average American diet.
Nutrients that are high in the American diet, such as fat, sugar,
salt, vitamin D, inorganic phosphate, proteins and more recently
supplemented calcium (Ca) and fiber, all increase the dietary
requirement of Mg.(26,27)
Recommended dietary allowances for adults:
The recommended dietary allowance is defined as: the amount of a
nutrient that maintains a balance of intake and output in healthy
adults, and is sufficient to assure health - as determined from
studies performed long enough for adjustment to be made to
altered intakes.(26) Analysis of worldwide metabolic balance
studies of free-living young adults, showed that when 4-<5
mg/kg/day of Mg (the content of typical American diets) is taken,
men tended to be in negative Mg balance, whereas women remained
in equilibrium(26). (Figure 1)
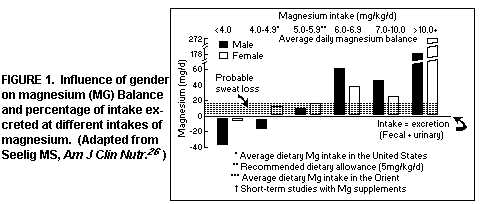
The analysis also disclosed that in countries (i.e. in the
Orient) where the Mg intake is higher (6>8 mg/kg/day), both
men and women were in positive Mg balance during the studies. The
decision that the recommended dietary allowance for Mg in the
stable adult should be 5 mg/kg/day(28) was derived from the
finding that maintenance of Mg equilibrium was unreliable on
lower intakes.(28)
However, many factors: dietary imbalance, normal anabolism as
during growth and development, and psychologic and physical
stress, including that of athletic training and competition, all
increase the need for Mg. Diseases and drugs that decrease Mg
absorption or increase renal Mg loss increase vulnerability to Mg
deficiency(27). (Figure 2)
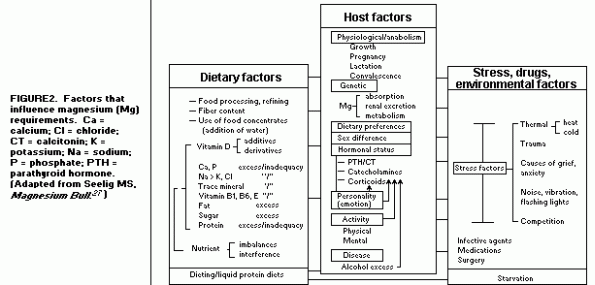
To assure adequacy of Mg to meet increased needs, the optimal
intake of adults may be 6-8 mg/kg/day.
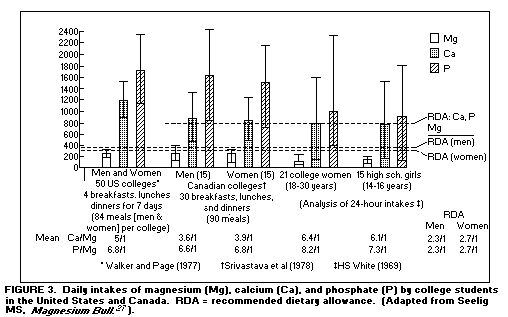
Dietary surveys - magnesium intakes below recommended
dietary allowance: Large scale dietary surveys have
disclosed that the dietary Mg intake of most Americans falls
below the recommended dietary allowance.(27,29-31) Vegetarians'
Mg intake is much higher, which could be a factor in their lower
incidence of cardiac and vascular disease. Self-selected meals of
students in American and Canadian high schools and colleges were
found to provide less than the recommended dietary allowance for
Mg, but more than the recommended dietary allowances for Ca and
phosphate(27,32-34). (Figure 3)
Diets high in saturated
fat:
Overconsumption of saturated fat, which is accepted as a key
factor in the high morbidity and mortality from cardiovascular
disease, increases the need for Mg. Mg deficiency alone has been
shown to cause cardiac and arterial pathologic changes in every
species of animal in which it has been induced.(1,13,35-38) High
fat/low Mg intakes appear to be conjoint pathogenic factors. The
arterial lesions of high fat diets in several species are
intensified by simultaneous Mg deficiency, and protected against
by Mg repletion.(1,13,35-42)
Fats form soaps with Ca and Mg, in the gut, thereby decreasing
absorption both of fat and divalent cations. However, the
protection afforded by high oral Mg intake, against both
hyperlipidemia and cardiovascular lesions, entails more than
diminution of fat absorption. Mg supplementation decreases the
enhanced thrombogenesis caused by high fat intake(36,43) (see
later).
Recent demonstration of the importance of Mg in fat metabolism
in rats(36-38,44,45) suggests that increased Mg intakes may also
exert a favorable influence on adverse effects of diets too high
in fat, even in humans. The increase in low-density lipoproteins,
very low density lipoproteins, and decrease in high-density
lipids seen in Mg deficient rats - on a diet providing 20%
casein, that was relatively low in fat (5% corn oil), but high in
sucrose (70%) (Table III),
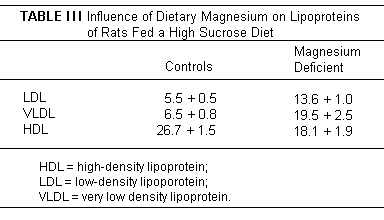
was reversed with Mg supplementation.(38,44) Triglyceride
plasma levels were also higher in Mg deficient rats than in
controls (same diet, but with adequate Mg) - an effect that was
shown to be caused by its impaired clearance.(38,44,45) A marked
decrease in esterified cholesterol (but no change in total
cholesterol) was reported in Mg deficient dogs on a high butter
fat diet in a 1933 study.(46) Fifty years later, the reduction in
esterified cholesterol of rats maintained on the aforementioned
high sucrose/5% oil/Mg deficient diet was shown to be caused by
decreased activity of lecithin-cholesterol acyl-transferase.(38)
It may be the subnormal lecithin-cholesterol acyl-transferase
activity that is responsible for the depression of high-density
lipoproteins in Mg deficiency - the high-density lipoproteins
being the major substrate for esterification by this
enzyme.(47)
A pilot clinical study of the effect of oral supplements of
MgCl2 on blood lipoproteins of 16 patients with abnormally low
high-density lipoprotein/very low density + low
densitylipoprotein ratios, showed that a mean daily dose of 17.9
mM for a mean period of 118 days significantly raised the
ratio.(48)
Sugar: These animal studies showing the
effects of Mg deficiency on lipoproteins were with high sucrose
diets.(36-38,44,47) In humans, sugar loading causes
magnesiuresis,(49) possibly converting a marginal intake to a
deficient one. Diabetes mellitus, which causes Mg wasting,
especially during periods of decompensation,(50,51) is marked by
hyperlipidemia and lesions of small blood vessels,(52,53) that
resemble those seen in experimental Mg deficiency.(1,13)
Calcemic Agents and Phosphates: Among the
other nutrients, of which the usual diet is likely to provide
more than is required, and excesses of which increase Mg needs
and are cardiovasopathic, vitamin D is of interest as both a
calcemic and hyperlipidemic sterol,(54-59) that has been shown to
be a steroid hormone.(60) Its requirements are individually
variable, and the amount provided to prevent rickets (in milk, by
addition to many foods, and in multivitamins), is enough to cure
rickets, even in those with high requirements, and is close to
the toxic dose for those whose need is low.(56-58) Vitamin D is
often one of the components of experimental cardiovasopathic
diets against which Mg is protective.(61) Inorganic phosphate
which is plentiful in the American diet (though cola beverages
and processed foods) is another component of cardiovasopathic
regimens.(16,62) Excess Ca is included in some of the
cardiovasopathic models, and elevated myocardial Ca is
characteristic of cardiac damage.(1,13,14) Finland, with the
world's highest dietary Ca/Mg ratio, has the world's highest
cardiovascular morbidity and mortality and incidence of sudden
death among young men.(16,63) A similar ratio may become a
problem in the United States, where Ca supplementation is
increasing. Similarly, unsupervised excess intake of fiber
(organic phosphate), which can reduce availability of Mg, as well
as of other divalent cations, may require mineral supplementation
to correct fiber-induced losses.(27)
Alcohol: Ethyl alcohol increases Mg needs,
even when taken in moderate quantities, through its enhancement
of renal Mg excretion.(64-66) The efficacy of Mg treatment of
neuromuscular and cardiac disorders of alcoholism was early
attributed to correction of the Mg deficiency.(67) Chronic
alcoholics become profoundly Mg depleted as a result of long-term
magnesiuresis, superimposed on poor intake; those with cirrhosis
also lose Mg as a result of secondary aldosteronism.(68,69)
During the stress of alcohol-withdrawal, the fatty acids released
as a result of catecholamine-induced lipolysis magnify the
problem by binding serum and cellular Mg.(70) Unless the
concomitant Mg deficiency is repaired during treatment of
alcoholism (which entails repair of thiamin deficiency) there may
be faulty response to administered vitamin B1 because of the
Mg-dependence of thiamin-activated enzymes.(71-74) In thiamin-
and Mg deficient rats, thiamin administration without correction
of the Mg deficiency intensifies tissue Mg depletion.(73)
Pregnancy, growth and development: Studies
show that Mg intake in pregnant women has been falling, while
their intakes of Ca, phosphate, and vitamin D have risen(1).
(Figure 4)
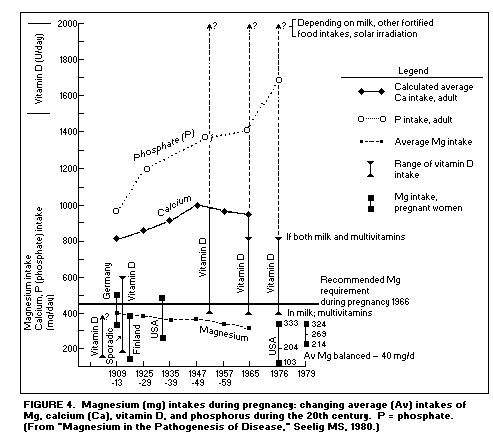
The lowest reported Mg intake and repeated negative Mg
balances during a long-term study of a pregnant woman were in
Finland,(75) (the country with the lowest Mg intake and the
highest Ca/Mg dietary ratio, as well as the highest
cardiovascular morbidity and mortality rates in relatively young
men.(16,63) Mg intakes of white, middle class pregnant American
women have disclosed that low dietary Mg results in negative Mg
balance during pregnancy.(76,78,79) This is a potentially
dangerous situation during a time when new tissue formation in
mother and fetus mandates an adequate supply. It is possible that
Mg deficiency may be a factor in peripartal cardiomyopathy and
arrhythmias, which often occur in women with conditions that
increase Mg requirements: maternal immaturity, multiple births,
high parity especially when rapidly successive, and diabetes
mellitus. The lesions resemble those of Mg deficiency.(1) That Mg
inadequacy during pregnancy might cause fetal arterial injury is
suggested by reports of coronary arterial lesions and generalized
arteriosclerosis, detected at autopsy in infancy and
atherosclerosis and cardiac damage early in childhood.(1,59) A
few studies of Mg requirements of very young children and
adolescents suggest that Mg needs during growth and development
are higher than they are in adults. Might inadequate Mg intakes
at times of high need contribute to cardiovascular disease?
Aging: The Mg intake of the elderly tends to
be low, and their susceptibility to Mg deficiency is intensified
by diminished intestinal absorption and increase urinary output
of Mg.(80-83) Elderly persons, who are subject to disorders that
impair absorption and renal function, and who may be taking
Mg-wasting medications, are likely to be particularly vulnerable
to Mg deficiency.(80)
Whether long-standing Mg deficiency affects the health of the
aged, and increases their susceptibility to adverse cardiac
reactions to disease and drugs that cause Mg-wasting deserves
investigation. Transient ischemic cerebral attacks, to which the
elderly are particularly prone, might be intensified by Mg
deficiency. Subjects who have Mg added to their diets have been
reported to be less likely to have transient ischemic
events,(84)
That the tolerance of old subjects to stress might be improved
by Mg supplementation is suggested by a study of rats maintained
on diets low in Mg from the time of weaning to death from old
age.(85) They adapted to the deficiency and ceased to show the
acute signs, that occurred at the outset (which caused death in
convulsions in some). However, the Mg-depleted survivors had
shorter lives and died with cardiac damage under conditions of
stress tolerated by controls.
Stress: A variety of stresses, both
psychological and physical, increase Mg requirements and cause
increased cellular Mg loss(27). (Figure 5)
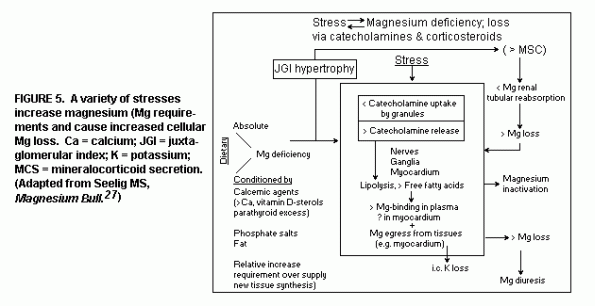
Stress and Mg deficiency are mutually enhancing. Experimental
Mg deficiency has been shown to cause hypertrophy of the
juxtaglomerular index,(86) which results in mineralocorticoid
secretion, which in turn increases Mg loss.(69,87) In vitro
studies have shown that catecholamine secretion is increased by
low Mg/Ca ratios, and decreased by high Mg/Ca ratios in
suspensions of catecholinergic cells of the adrenal cortex(88)
and of ganglionic nerve endings(89. (Figure 6)
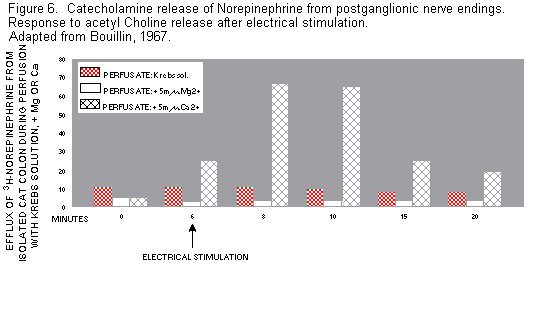
In vivo rat studies have shown that hypomagnesemic
noise-stressed rats excrete more catecholamines than do
control-fed stressed rats.(90,91)
A vicious cycle can thereby develop, because exogenous or
endogenous catecholamines secreted as a result of stress cause
mobilization of cellular Mg, particularly from the myocardium -
from which 12-39% losses have been reported, in association with
uptake of Ca.(91-96) The loss of myocardial Mg precedes cardiac
damage and Ca accumulation.(14,93-95) It has been postulated that
catecholamines also block Mg ingress across a proposed
Mg-channel.(97,98) This theory is in accord with data suggesting
that catecholamines block Mg-uptake via a Mg-channel by lymphoma
cells.(99) The highly protective effect of verapamil, the
Ca-channel blocking agent, against catecholamine-induced cardiac
necrosis, has been associated both by reduction of Ca-uptake and
of Mg-loss.(92,97)
These effects may explain the transitory increase in serum Mg
that has been shown in noise-stressed rats and in guinea pigs
stressed by overcrowding. They exhibited decreased erythrocyte Mg
and increased serum Mg that derived from released intracellular
Mg, and then increased urinary excretion of Mg.(90) The
longer-term fall in Mg levels during the stress of exposure to
cold of ruminants transferred from barn to early spring
pasture,(100) alcohol withdrawal,(70) and myocardial
infarction(101) has been attributed to stress hormone induced
release of fatty acids, with resultant inactivation of Mg in the
serum and tissues. Another interpretation is that epinephrine,
which increases Mg-binding in adipocytes,(102) a reaction that is
coupled to adrenergic lipolysis,(103) might be responsible for
the reduction in serum Mg.
Subjects with Type A personalities, who have increased urinary
catecholamines and circulating free fatty acid levels, been shown
to have lower erythrocyte Mg levels than do those with more
phlegmatic Type B personalities, who are less vulnerable to
stress-related cardiovascular disease.(104-107) Patients with
latent tetany associated with Mg deficiency - may be especially
vulnerable to stress-induced catecholamine release. This
condition is diagnosed more frequently in Europe than in the
United States, and has received detailed consideration.(108)
Whether those presenting with nervous complaints, with or without
latent tetany, might be especially vulnerable to heart disease as
a result of catecholamine induced increase of Mg requirements (as
are Type A subjects) is moot. Among (130) children with "nervous"
complaints related to psychosocial and school stresses 16% had
hypomagnesemia, and 41.5% had hypocalcemia; 62% improved with
oral Mg aspartate HCl supplementation.(109) Among 842 children,
diagnosed as having latent tetany and respiratory alkalosis, who
had comparable complaints, satisfactory improvement was achieved
in 56% by oral treatment with MgCl2, and there was partial
amelioration in 32%.(110)
Stresses to which normal young people are subject include
those experienced during competition (athletic and
career-oriented), crowding and subjection to flickering lights
and vibration - as in subways or discoteques. Those with genetic
predisposition to poor tolerance of psychological
stress,(104-109), or others exposed to physical or psychological
stress, are at particular risk of Mg inadequacy.(1,27,74,78) The
close interrelations of suboptimal Mg intake, coupled with the Mg
loss induced by excess release of stress-hormones, suggest the
desirability of Mg supplementation, as a reasonable means of
reducing the risk of cardiovascular disease culminating in
chronic disease, myocardial infarction, or sudden unexpected
death from cardiac arrhythmia.
Histologic
Lesions:
Diets delivering amounts of Mg sufficient to prevent death in
experimental animals but insufficient for optimal growth and
development, have caused arterial and cardiac lesions, the nature
of which differs depending on other dietary factors.(1,13,35) In
animals fed Mg deficient, but otherwise acceptable diets, early
myocardial lesions develop predominantly in the small coronary
and intramyocardial arteries.(1,13,35,111,112) There are
pathologic sarcosomal and mitochondrial alterations, with Ca
accumulation before the cell dies and multifocal necrosis
develops.(111,112) There are many Mg-dependent processes in the
heart that entail interactions with Ca.(10,11) Mg modulates Ca
uptake by myocardial mitochondrial,(113,114) thereby protecting
against conditions and drugs that increase Ca ingress and damage
to the heart.(1,93,113,114) The lesions of the mitochondria of
the heart caused by Mg deficiency resemble those of myocardial
ischemia and of catecholamine induced cardiopathy; in
experimental models, and in fatal clinical ischemic heart
disease, the first alteration is loss of Mg from the
heart.(1,13,14,93,95,111,115-119)
Intensification of Atherogenesis by Mg
Deficiency: Atherosclerosis is frequently produced by
feeding animals diets high in fat/cholesterol. The Mg deficiency
induced lesions of the small and medium sized arteries in several
animal species are characterized by edematous and thickened
intima, and thinned, split, and fragmented internal elastica
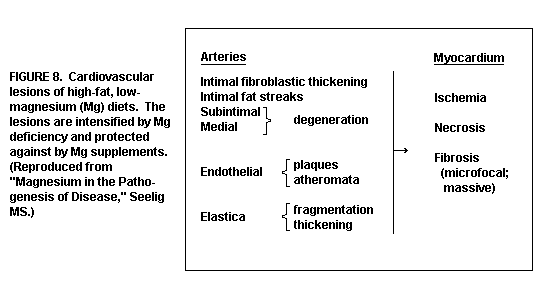
sometimes with lipid droplets(1,13,35). (Figure 7)
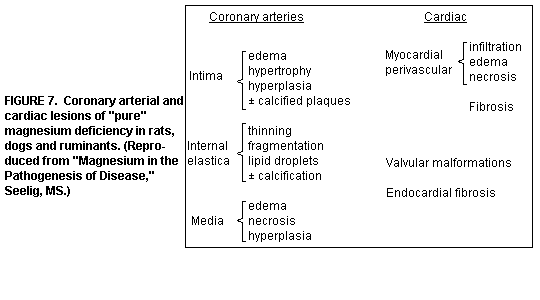
Coronary arteriopathy: hyperplasia of smooth muscle cells,
fibrinoid necrosis, and chronic medial and adventitial
inflammation develop in the hamster, a rodent that is
particularly vulnerable to Mg deficiency, when fed a Mg deficient
diet.(120) When, in addition to low Mg the diet includes excesses
of saturated fat and/or calcemic agents, or both, larger arteries
are affected, and atherosclerosis develops(1). (Figure 8)
(Figure 9)
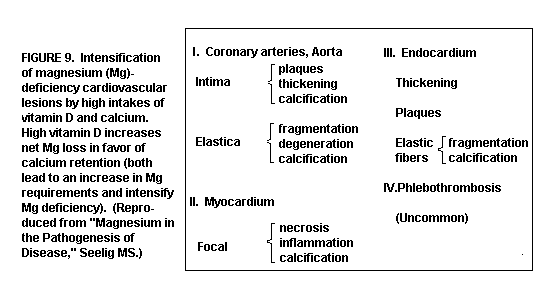
The lesions are intensified over those seen in the absence of
Mg deficiency.(1,13,35,39-41)
Coagulation, and Thrombosis: The possibility
that early lesions of atherosclerosis are mediated by
organization and fatty changes in mural thrombi at sites of
arterial damage(121) calls attention to the possible role of Mg
deficiency, which increases intra-arterial coagulation. Mg
deficient animals had significantly shorter thrombin clotting
time than did controls.(122) Hypercoagulability caused by a
thrombogenic diet rich in fat was counteracted by oral
supplements of MgCl2.(43) Most of the in vitro studies showing
that Mg inhibits coagulation factors: prothrombin, thrombin, and
Factors V, VII, and IX, have been with unphysiologically high Mg
concentrations.(1,108)
The anti-thrombotic effect of high concentrations of Mg has
been investigated in animals receiving standard diets, with
artificially induced intimal lesions. The suppression of platelet
aggregation by local application of a MgSO4 solution (6%) was
demonstrated at areas of arterial injury caused by holding or
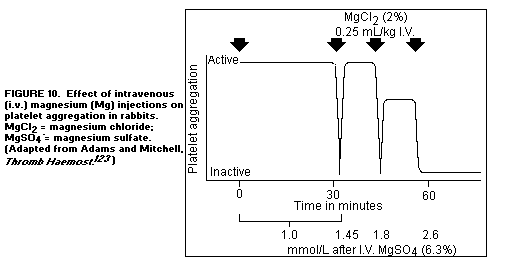
pinching with a forceps.(123) Repeated intravenous infusions of
isotonic MgCl2 also suppressed thrombus formation(123). (Figure
10)
The effect of high doses (50 mg/kg) of intravenously
administered MgSO4 (25%) on platelet deposition in arteries with
suture ligation of arteries causing 40-60% constriction has been
reported.(124) (Dogs) with a narrowed major coronary artery, and
rabbits with a narrowed carotid artery, pretreated with Mg,
showed marked diminution of platelet aggregation at the site of
constriction, and absence of microthrombi in the arteries distal
to the ligation. It has been suggested that these findings have
potential therapeutic or prophylactic implications - in
Prinzmetal's variant angina where arterial spasm is the principal
factor, and in coronary thrombosis.(124) There is some evidence
that these findings may be applicable to the clinical
situation.
Patients with thromboembolic disease, associated with latent
tetany, have responded to Mg therapy by correction of
hypercoagulability caused by excess adenosine diphosphate-induced
platelet aggregation.(125,126) Increased platelet aggregability
has been demonstrated in myocardial infarction patients, in
association with decreased serum Mg levels.(127) In addition, Mg
therapy has yielded benefit in patients with Prinzmetal
angina(128) and in post-myocardial infarct patients (see
later).
Fibrotic Changes in Thrombi and Myocardium:
Fibrosis constitutes a large portion of the occlusive thrombi,
examined from cases of fatal myocardial infarction.(129). Since
increased fibrosis and slowed collagen resorption has been
reported in tissues damaged by experimental Mg deficiency,(36,37)
it is plausible that Mg deficiency may be a factor in clinical
cardiomyopathies. Fibrotic cardiomyopathy has been associated
with abnormalities during the perinatal period (in mother as well
as infant) alcoholism, protracted diarrhea, protein calorie
malnutrition, in hypercalcemic states, such as
hyperparathyroidism and vitamin D hyperreactivity or toxicity,
and in congestive heart failure.(130) All are conditions
associated with Mg deficiency, as a result of the disease, or of
treatment.
Myocardial Ischemia of Coronary Insufficiency or
Occlusion: Ischemic heart disease, which is responsible
for about a third of all deaths in the Western world, is caused
by coronary atherosclerosis with or without thrombosis that gives
rise to myocardial infarction, arteriospasms, and resultant
arrhythmias. Many theories have been propounded as to the
pathogenesis of this widespread disease-complex.(118,131)
Inadequacy of Mg is a common denominator in some of the proposed
mechanisms. The cited damaged intima, and lipid infiltration of
Mg deficient animals, and their increased blood coagulability
bear on the endothelial injury/lipid infiltration/mural
thrombosis theory of atherogenesis.(132) The cited role of Mg in
reversing low high-density/high-density ratios may also be
germane to that theory. The importance of optimal Mg levels in
counteracting arteriospastic agents - neurohormonal and
electrolyte ultimately affects angina pectoris and peripheral
arterial spastic disease.(118,119)
Pertinent to the effect of Mg on the damage to the myocardium
caused by coronary occlusion, are the demonstrations in rats that
its extracellular depletion causes impaired postischemic cardiac
function and metabolism,(133) and its dietary inadequacy in dogs
results in increased infarct size after coronary artery
occlusion.(134) It has been proposed that the protective effects
of Mg during myocardial ischemia entails not only its limiting
the loss of cellular Mg and K, and militating against
Ca-overload, but restricting the cellular loss of Mg-adenosine
triphosphate the essential substrate for many cellular
reactions.(135-137) The experimental findings suggest that early
myocardial changes after an ischemic event (perhaps during the
first 20-30 minutes) might be reversible, and that applying the
findings to patients with a recent MI might result in better
preservation of the ischemic myocardium and limitation of the
size of the infarct.(137)
Electrophysiologic Cardiac Findings: With Mg
deficiency severe enough to produce convulsions in rats, the
electrocardiogram first disclosed tachycardia, followed by marked
arrhythmia and then bradycardia just before the seizures
started.(46) During post-convulsive unconsciousness, a
sinoauricular block developed, with occasional skipped or ectopic
ventricular beats.(138) In young dogs with average serum Mg of
0.4 mEq/liter, the PQ interval and QRS were markedly shortened,
with lesser shortening of the QT interval, and an increased
incidence of negative T waves.(139) With a semisynthetic diet
that lowered the serum Mg to < 0.5 mEq/liter without seriously
affecting serum K or Ca, there was sinus tachycardia, but little
change in PR, QRS or QT intervals.(140) Less severe Mg deficiency
in dogs and monkeys produced changes like those of extracellular
hyperkalemia (as K shifted out of cells): marked sinus
tachycardia, peaking of T waves, and ST depression.(41,141)
Electrocardiographic similarities among magnesium,
potassium and calcium abnormalities - experimental and
clinical: The electrocardiographic changes of Mg
deficiency differ, depending on the degree of the deficiency
which further influences K and Ca intracellular and extracellular
levels. Therefore, it is not surprising that there are
similarities between the electrocardiograms of Mg deficiency and
those of Ca or K abnormalities(1,142), (Figure 11).
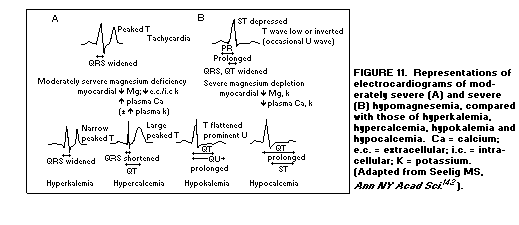
The loss of myocardial K (with transitory elevation of
extracellular K) that results from myocardial Mg loss can
contribute to electrophysiologic changes of Mg deficiency that
resemble that of moderate hyperkalemia: a T-wave that is peaked
but narrower than that of Mg deficiency.(1,142,143)
The common pushing of K therapy to overcome the (often
undiagnosed) hypomagnesemia-associated refractory
hypokalemia(21,22) can intensify Mg deficiency(144) and be
associated with the electrocardiogram of K-depletion. With
long-standing Mg deficiency, the electrocardiogram with its
flattened T-wave (and sometimes a U-wave) is more similar to that
of hypokalemia, with which it is associated.(142-144)
Early hypomagnesemia can be accompanied by hypercalcemia that
may be caused by transient hyperparathyroidism(145) and that
might contribute to the broader T wave peak (than that of
hyperkalemia) seen with moderate Mg deficiency. More severe Mg
deficit is associated with hypocalcemia, in association with both
impaired release of parathyroid hormone and blunting of the
target organs to its action.(145) At that stage, the
electrocardiogram of Mg deficiency also resembles that of Ca
deficiency. However, at that stage, there is a shift of Ca into
the myocardium. Much of the protective effect of Mg against
arrhythmic stimuli is being attributed to its being a physiologic
Ca blocker.(118,119,146)
Many of the clinical conditions, in which electrocardiographic
changes of hypomagnesemia have been reported, have been
associated with electrocardiograms characteristic of hypokalemia
or hypocalcemia. Basic to the role of Mg in maintaining or
restoring cardiac rhythmicity is its role in maintaining K and Ca
homeostasis. In brief, the electrocardiogram associated with
clinical hypomagnesemia is usually characterized by increased
sino-atrial discharge rate, with a tendency towards abnormal
impulse formation, and ventricular ectopic beats.(8,147)
Prolongation of the PR and QT intervals reflect the
arrhythmogenic potential of Mg deficiency. Widening of the QT may
indicate a lengthened repolarization phase, caused by altered
membrane transport of K, associated with aberrant conduction,
reentry, and lowered threshold for ventricular fibrillation.
Arrhythmias: The efficacy of intravenously
administered Mg in control of clinical arrhythmias has been
reported at intervals during the past half century. Since the
early 1970s when this effect was associated with correction of Mg
deficiency, (6,7) a variety of dysrhythmias incompletely
responsive or refractory to conventional treatment have been
controlled by parenteral Mg therapy (Table IV).(8)
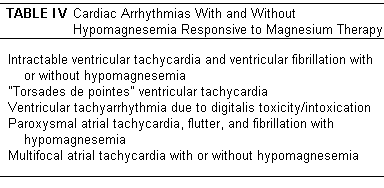
Although the Mg-responsive arrhythmias are often not
associated with low serum Mg levels, better tests for Mg
deficiency(42) have shown it to be implicated in the conditions
in which cardiac dysrhythmias develop.(6-9,148-159)
Dysrhythmias responsive to Mg occur in alcoholism -
particularly during alcohol withdrawal,(6,8,64,65,154-159) during
long-term administration of parenteral fluids lacking or
inadequate in Mg, or in those undergoing postsurgical drainage of
gastrointestinal fluids, or a combination of all
3.(1,65,154,160-162) They have been reported in patients with Mg
deficiency caused by chronic diarrhea or short bowel.(7,160-163)
The diarrhea of protein calorie malnutrition can be a
predisposing factor to the arrhythmias seen in victims of
starvation, especially during refeeding with formulas inadequate
in Mg.(164).
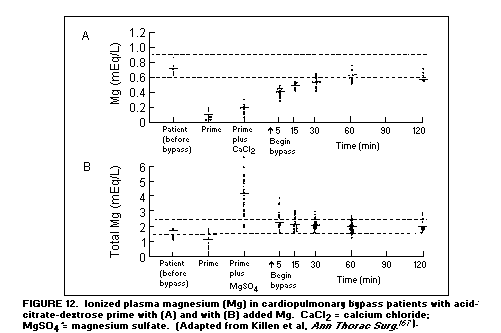
The arrhythmias during exchange transfusions(165,166) and
after open-heart surgery are partially caused by hypomagnesemia
resulting from the use of anticoagulants such as
acid-citrate-dextrose, that bind Mg as well as Ca (only Ca
usually being repleted)(167). (Figure 12)
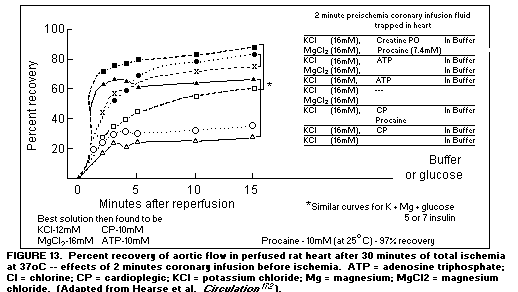
The use of Mg in the postcardiotomy period has decreased the
incidence of arrhythmia.(168-171) Addition of Mg to cardioplegic
solutions used in the pump-prime during cardiac surgery has
proved to be the single most effective component of the
protective infusates tested(172), (Figure 13)
Myocardial Infarction: Treatment of
myocardial infarct patients with parenteral Mg salts was reported
to decrease arrhythmias and improve survival in uncontrolled
studies in South Africa, Australia and Europe from 1958 through
the 1960s.(1) Recent controlled studies have verified the
improvement in management and survival after acute myocardial
infarction achieved by intravenous Mg therapy.(173-177) There is
growing evidence that Mg deficiency may be a predisposing factor
for myocardial infarction and subsequent
complications,(8,23,91,147-153,178) Addition of Mg to the
postmyocardial infarction regimen parenterally in the early
phase, and orally subsequently - needs serious consideration.
Congestive Heart Failure: The most
arrhythmogenic disease, congestive heart failure,(179-182) is
responsible for many unexpected sudden deaths - not from
progressive circulatory failure, but suddenly and unexpectedly,
at a rate even higher than among patients in the first 12 months
after myocardial infarction.(179) This has been attributed to the
dysrhythmias caused by the electrolyte disturbances produced both
by compensatory mechanisms and by treatment of the disease.
(Figure 14)
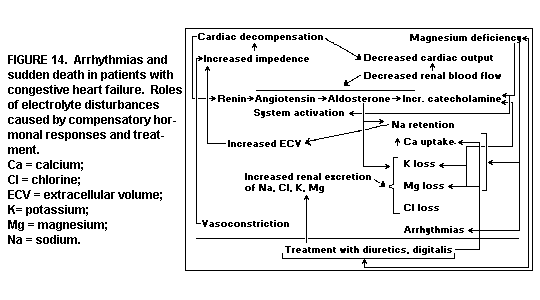
The compensatory mechanisms resulting from reduced cardiac
output cause increased secretion of vasoconstrictor and volume
regulating hormones: catecholamines,
renin-angiotensin-aldosterone, and anti-diuretics,(180,183)
Catecholamine and aldosterone secretion is increased by
underlying Mg deficiency - which is increased by both diuretic
and digitalis therapy (see before), which further stimulate the
neurohormones.(180,183) Loss of K and Mg, caused by diuretics and
aldosterone, increases arrhythmias, which are intensified by
angiotensin's stimulation of aldosterone secretion and
potentiation of the sympathetic nervous system.(180,183)
Correction of secondary aldosterone- andtreatment-induced
losses of both K and Mg is responsible for the favorable
immediate response of heart failure patients with digitalis
arrhythmias. When both cations are deficient, repletion of Mg is
necessary for the repair of Mg and K tissue levels and the
dysrhythmias.(23,180-182) Infusion of K before Mg infusion had a
much weaker anti-arrhythmic effect than did Mg infusion alone; in
several patients, the K infusion actually caused more ectopic
beats, that were largely corrected by the Mg infusion.(184)
Rapid electrolyte depletion might explain the precipitation of
lethal arrhythmias in patients with left heart failure with
myocardial fibrosis, ventricular wall stress and elevated
circulating neurohormones.(182) Hypokalemia is most often
recognized in this condition, and in patients with congestive
heart failure resuscitated after in-hospital cardiac arrest. It
has been suggested that the only antiarrhythmic intervention
needed to prevent recurrence of cardiac arrest may be repletion
of K and Mg.(182)
Mitral Valve Prolapse: The electrocardiogram
patterns and arrhythmias of mitral valve prolapse that are like
those seen in Mg deficiency(185) are those present in a condition
frequently seen in many patients with latent tetany of Mg
deficiency.(108,185,186) The finding of decreased red blood cell
Mg levels in patients with the human leukocyte antigen haplotype
HLA haplotype HLA-Bw35,(187) has been associated with mitral
valve prolapse,(188) supports the premise that there is a
relation. Additional theories as to how Mg deficiency might
participate in the pathogenesis of this disease have been
considered.(108,186)
Hypertension: Hypertension is the most
prevalent risk factor for premature cardiovascular diseases in
the United States; it is estimated that over 30 million are
affected.(189) Reviews of the dietary changes recommended for its
prevention and treatment summarize strengths and weaknesses of
past and current recommendations: caloric and sodium (Na)
restriction, and K, Ca, and Mg supplementation.(189,190) The
premise that dietary Ca deficiency is an important factor in the
pathogenesis of hypertension,(190) and the evidence as to the
proposed mechanisms that might be involved have been analyzed and
found questionable.(191)
Unlike Ca, high serum levels of which increase blood
pressure,(191) hypermagnesemia produced by Mg administration in
pharmacologic doses has long been found efficacious in the
treatment of severe hypertension, as in toxemias of
pregnancy,(2,3) There is substantial experimental evidence that
dietary Mg deficiency increases arterial contractility, and that
elevating Mg decreases blood pressure.(1,118,192-194) However,
severe experimental Mg depletion reduces the activity of
vasopressive neurohypophyseal peptides.(1,192) This may explain
the hypotension of patients with renal Mg wasting.(195)
The fact that hypertensive patients with low plasma renin
activity (who have higher than average serum Mg levels) respond
to administration of Ca, while those with high renin activity
(who have lower serum Mg levels) respond to Mg treatment(196)
does much to clarify the roles of Mg and Ca in hypertension. Of
particular interest is the evidence of low levels of free Mg in
erythrocytes in both forms of hypertension.(197,198)
It was found that Mg infusions - in patients with congestive
heart failure and with hyponatremia, increased muscle Na and Cl,
and subnormal muscle K and Mg and with increased resistance to
diuretics - corrected the electrolyte abnormalities.(199)
Comparable results were obtained with oral Mg supplements in CHF
and hypertensive patients taking long-term diuretics.(200)
Digitalis:
The first demonstration of the anti-arrhythmic effects of Mg
was in the treatment of digitalis toxicity, more than 50 years
ago.(201) Almost 30 years later, low serum Mg levels (1.38
mEq/liter +/- 0.13) were found in patients with arrhythmias of
digitalis toxicity.(202) These findings have been repeatedly
confirmed.(5,203,204) Patients with normal serum Mg levels, but
decreased lymphocyte Mg, have also been shown to respond to
intravenous Mg therapy by correction of the arrhythmias.(205)
Hypomagnesemia has been reported more frequently than hypokalemia
in patients with digitalis toxicity.(206) Among patients with
symptomatic atrial fibrillation 20% were found to be
hypomagnesemic; these required twice as much digoxin to control
the arrhythmia as did those with normal serum Mg.(207)
The mechanisms by which Mg deficiency increases digitalis
toxicity and by which Mg administration protects against
digitalis arrhythmias, have been elucidated by animal and in
vitro experiments. In vitro studies of isolated calf cardiac
Purkinje fibers(208) suggested that excess digoxin poisons the
membrane Na and K-adenosine triphosphatase with resultant
inhibition of the Na-K pump, which is Mg dependent.(10,11). As a
result, there is a reduction in the intracellular K/Na ratio that
causes phasic movement of Ca in and out of the myoplasm, and
transient inward current and spontaneous depolarizations. Low Mg
levels potentiate these currents, while high Mg levels block
them.(8.208) A study of digitalis-induced arrhythmias in dogs did
not confirm the hypothesis that Mg reactivated digoxin-inhibited
Na-K adenosine triphosphatase, suggesting that Mg directly
affects Ca and K fluxes across the cell membrane.(209) A study of
the efficacy of repeated intravenous infusions of MgCl2 in intact
dogs given doses of digitalis that induced ventricular
tachycardia showed that Mg corrected the arrhythmia, or when
given before the digitalis, prevented it.(210)
Digitalis toxicity has also developed in patients with
congestive heart failure who had hypermagnesemia as a result of
concomitant renal impairment.(203) This seems contradictory to
the evidence that Mg deficiency increases digitalis toxicity.
However renal failure patients may have Mg deficiency, despite
their higher than normal serum Mg levels, as indicated by
subnormal skeletal muscle Mg concentrations.(211-213)
Diuretics: With regard to the risk of
arrhythmia, most classes of diuretics have long been known to
cause Mg as well as Na and K loss. Although the loop diuretics
are the main offenders in treatment of congestive heart failure,
patients receiving long-term treatment with such agents as the
thiazides, are at risk, especially the elderly,(214-216) Serum Mg
determinations are less reliable than tissue values.(42) In a
study of muscle and serum Mg and K levels in 34 patients in whom
ventricular ectopic beats developed while taking long-term
diuretic therapy, Mg infusions more effectively reduced the
ectopic beats than did K infusions.(184) In a larger study among
297 patients with congestive heart failure who had received
long-term diuretic treatment, serum Mg was subnormal in 37%, and
skeletal muscle Mg was subnormal in 43% (almost all of whom also
had low muscle K(23)).
The increase of mortality among those participants, receiving
diuretics, in large-scale anti-hypertension studies has called
attention to the possibility that Mg loss, as well as K loss
(which is usually watched for and corrected) may be at fault. Mg
loss, in contrast, is usually undetected. In the extensive
randomized Multiple Risk Factor Intervention Trial (MRFIT), that
was sponsored by the National Institutes of Health, there was
poorer survival in a subgroup who had had baseline
electrocardiogram abnormalities and had been treated with
thiazide diuretics, than in those not so treated.(217,218) In a
study of mildly hypertensive patients 165 of whom (n = 287) had
ambulatory electrocardiography, ventricular ectopic beats
occurred more frequently in thiazide-treated patients than in
placebo-treated controls.(219) A higher mortality rate due to
fatal myocardial infarction and sudden deaths had been reported
earlier in elderly hypertensive men, treated with diuretics, than
in comparable patients treated only with salt restriction or
beta-adrenergic blocking agents.(220) This is contrast to the
European double-blind multicenter study, in which the study group
was given diuretics plus Mg and Ksparers; survival of that group
of subjects was 15% better than it was in the control
groups.(221)
In two of the Multiple Risk Factor Intervention Trial centers,
the serum Mg levels of 300 participants, aged 35-57 years, were
analyzed after 4 to 5 years of treatment with relatively low dose
chlorthalidone or hydrochlorthiazide.(222) About 15% of these had
persistently lower Mg levels on 2 samples taken 4 months apart,
than did those not taking diuretics. The investigators noted that
the small differences might reflect the inadequacy of serum Mg as
an index of Mg loss, and that further study is indicated, using
better indices of the Mg status.
Hypochloremic metabolic alkalosis interferes with
potassium repletion: Diuretic-induced Cl loss is less
frequently noted than is hypokalemia, with or without Mg loss.
Metabolic alkalosis can develop, especially in patients with
congestive heart failure, but also appears in hypertensive
patients receiving long-term diuretics. In an update of
complications of thiazide diuretic therapy, in which the above
disappointing results in large scale antihypertensive
intervention trials were summarized, emphasis was placed, not
only on Mg and K losses, but on the metabolic alkalosis caused by
diuretic- and aldosterone induced Cl loss as a contributory
factor in intracellular K depletion, and refractoriness to K
repletion.25 Patients with metabolic alkalosis, associated with
volume contraction and Cl loss induced by diuretics and secondary
aldosteronism, require large doses of KCl to repair the cellular
K deficit. A patient with renal Mg wasting developed hypokalemic
hypochloremic alkalosis while being treated with a diuretic for
edema of unknown derivation, despite administration of KCl.(195)
Only when MgCl2 was added was normal electrolyte status
restored.
Reports, in which the importance of Mg in repleting
intracellular K is considered, sometimes refer to the concomitant
existence of metabolic alkalosis, that intensifies K loss, but
usually only in passing.(23,180,181,204) Problems of metabolic
alkalosis are usually considered in reports on acid/base
disorders.(24) The diuretics in common use promote cation
excretion almost exclusively in association with Cl. Because
patients receiving diuretics are often advised to consume low
salt diets, that are thus also low in Cl, metabolic alkalosis -
often not diagnosed - may exist. At first, the alkalosis was
considered the result of K depletion; however, subsequent studies
showed the K deficit often encountered in metabolic alkalosis (as
high as 300 to 500 mEq) is a consequence of the alkalosis rather
than its cause.(24) In 1955, acute metabolic alkalosis induced by
removal of Cl was shown to increase K excretion in rats.(223) In
a study of dogs made alkalotic by a diet low in K and Cl, and by
administration of a mineralocorticoid and sodium bicarbonate, Cl
was shown to be critical in repair of alkalosis.(224) Full
correction of the hypokalemia required sufficient Cl to repair
the hypochloremia. The importance of Cl in repair of hypokalemic
alkalosis in humans was then shown by balance studies performed
in normal human volunteers with alkalosis induced by Na nitrate,
and in patients with diuretic-induced metabolic alkalosis.(225)
Both in the experimentally induced and the diuretic induced
alkalosis, liberal intakes of K had no effect on plasma
bicarbonate and little effect on body K, if the Cl deficiency was
not corrected.
One of the patients with metabolic alkalosis studied by
metabolic balance(225) had chronic obstructive pulmonary disease.
A series of such patients, who had a tendency towards metabolic
alkalosis, rather than respiratory acidosis, had multifocal
atrial tachycardia that responded to Mg sulfate infusions.(226)
Four of the 9 patients with chronic pulmonary disease had serum
Mg levels below 1.5 mEq/ liter and 5 had levels higher than 1.8.
Even those with normal serum Mg retained substantial amounts of
infused Mg, indicating deficiency. Possibly, their respiratory
insufficiency might have caused sufficient hypoxia to mobilize
tissue Mg, raising serum levelsas has been shown to result from
too long application of a tourniquet, while withdrawing blood
samples,(227), and as has been seen in birth asphyxia.(228)
Alkalotic patients had greater vulnerability to digitalis
toxicity, even though they had therapeutic digitalis blood levels
lower than the nonalkalotic patients.(229) They improved with KCl
treatment, even though they were normokalemic, an effect that was
attributed to correction of decreased intracellular K that had
been caused by metabolic alkalosis. Their serum Mg levels were
not reported.
Risk of intensifying alkalosis by repletion of non-Cl
salts: Among the studies that showed that Cl is critical
to repair K loss and alkalosis, were case studies that showed
worsening of alkalosis when buffered K rather than KCl was
provided,(225) In another instance, a patient who abused Mg
cathartics, developed hypochloremic, hypokalemic metabolic
alkalosis, believed to have resulted from conversion of the Mg
oxide to MgCl2 in the stomach, and fecal excretion of endogenous
Cl.(230)
Animal studies of the intestinal absorption of different Mg
salts have shown a tendency towards hypochloremic alkalosis with
all of the Mg organic compounds and inorganic salts, except those
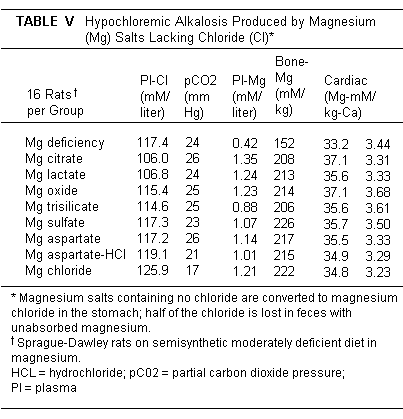
containing Cl (Table V).(231)
Rats fed MgCl2 had a slight tendency towards hyperchloremic
acidosis, which might be useful in subjects with diuretic induced
hypochloremic alkalosis.
Improvement in intestinal absorption of Mg, and correction of
extracellular alkalosis, with use of Cl-containing Mg compounds
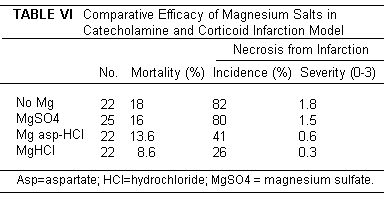
was credited with the greater cardioprotective effect of MgCl2
and Mg aspartate hydrochloride in adrenergic cardiopathy (Table
VI).(231-234)
The experimental model employed a mixed
gluco-mineralcorticoid, that is a very potent agent for inducing
hypochloremic alkalosis and marginal Mg deficiency, and that in
combination with epinephrine or with dietary Mg deficiency, or
both, lowers the myocardial Mg and raises the myocardial Ca
before development of necrosis.(234) Feeding a Cl containing Mg
compound (Mg aspartate hydrochloride) protected against cardiac
electrolyte changes and necrosis.
Early studies of the electrolyte-steroid-cardiac necrosis and
stress rat models showed that MgCl2 and KCl were the most potent
protectors against cardiac damage.(62) In a comparative study of
old and young stressed rats, it was found that Cl-deficiency
particularly intensified the risk of cardiac necrosis in old
rats.(235)
Repair of intracellular potassium deficits associated
with deficiencies of magnesium and chloride: Most of the
foregoing data deals with evidence that when Mg and K
deficiencies coexist, repletion of Mg is necessary for repair of
the cellular K loss. Since hypochloremic alkalosis has also been
shown to interfere with K repletion, it seems appropriate to
provide Mg and Cl, as well as the K that is usually provided, to
patients with diseases and those subjected to treatments that
cause loss of all 3 ions (Table VII).
Because of the hygroscopic nature of MgCl2, it must either be
supplied as a solution for oral use or in protectively coated
tablets.
In addition to the response of a patient with Mg-deficient
latent tetany to elixir of MgCl2,(195) there is a report of
satisfactory improvement of most of 842 children diagnosed as
having latent tetany and respiratory alkalosis on treatment with
orally administered MgCl2.(110) There are several preliminary
reports on the efficacy of an enteric coated MgCl2 preparation in
cardiovascular disorders.(48,236,237) Doses of 4-6 tablets, each
containing 0.5 g MgCl26H20, given for 6 weeks to 2 years, were
effective in decreasing QTc and QUc intervals in 25
patients.(236) The same preparation was found to improve the
antihypertensive response to a thiazide diuretic of 21
hypertensive patients aged 42 to 82 years.(237) Abnormally high
low-density lipoprotein levels were reduced in 16 patients given
6-10 coated MgCl2 tablets daily for a mean period of 118
days.(48)
There is substantial experimental evidence of the vital role
of Mg in maintaining cardiovascular integrity and normal
function. Large-scale dietary surveys have shown that American
diets are usually Mg deficient, inadequately meeting requirements
under conditions of growth and development, stress, or disease
and drug therapy that cause Mg loss. Mg has long been used for
parenteral treatment of convulsions and hypertension of
eclampsia, and more recently, as a therapeutic modality in
refractory cardiac arrhythmias (although usually as a last
resort). Mg has potential value in the management of
cardiovascular diseases treated with Mg-wasting drugs that
intensify Mg deficiency. Such diseases and treatment also
predispose to Cl loss induced metabolic alkalosis, which with Mg
deficiency, contribute to refractory cellular K depletion.
The fact that chronic Mg deficiency is silent and difficult to
diagnose - serum Mg levels being an unreliable index of the
cellular Mg status, has militated against early treatment or
supplementation with Mg. Studies should further assess the Mg
status in persons with conditions that may cause Mg deficiency or
those being treated with Mg- and K-wasting drugs. All of the ions
that are lost should be repleted - Mg, K, and Cl (to prevent or
correct metabolic alkalosis). It is further proposed that optimal
Mg intake throughout life, and especially under conditions of
normal anabolism and stress, may reduce the risk of
cardiovascular disease, and even of sudden unexpected cardiac
death.
1. Seelig MS. Magnesium Deficiency in the Pathogenesis of
Disease. Early Roots of Cardiovascular, Skeletal, and Renal
Abnormalities. Plenum Publ Corp, NY, 1980.
2. Lazard EM. A preliminary report on the intravenous use of
magnesium sulphate in puerperal eclampsia. Am J Obst Gynec
1925;26:647-656.
3. Pritchard JA. The use of magnesium in the management of
eclamptic toxemias. Surg Obst Gynec 1955;100:131-140.
4. Boyd LJ, Scherf D. Magnesium sulfate in paroxysmal
tachycardia. Am J Med Sc 1943;206:43-48.
5. Szekely P. The action of magnesium on the heart. Brit Heart
J 1946; 8:115-124.
6. Iseri LT, Freed J, Bures AR. Magnesium deficiency and
cardiac disorders. Am J Med 1975;58:837-846.
7. Chadda KD, Lichstein E, Gupta P. Hypomagnesemia and
Refractory arrhythmia in a nondigitalized patient. Am J Cardiol
1973;31:98-100.
8. Iseri LT. Magnesium and Dysrhythmias. Magn Bull 8: 223-229,
1986.
9. Packer M, Gottlieb SS, Kessler PD. Hormone-electrolyte
interactions in the pathogenesis of lethal cardiac arrhythmias in
patients with congestive heart failure, basis of a new
physiologic approach to control of arrhythmia. Am J Med 1986;80
(Suppl 4A):23-29.
10. Wacker WEC. "Magnesium and Man", Harvard University Press,
Cambridge, MA 1980.
11. Aikawa JK. "Magnesium: Its Biologic Significance", CRC
Press, Boca Raton, FL, 1981
12. Green DE, Fleischer S. The mitochondrial system of
enzymes. in "Metabolic Pathways", Academic Press, 1980
13. Seelig MS, Heggtveit HA. Magnesium interrelationships in
ischemic heart disease. Am J Clin Nutr 1974;27:59-79.
14. Lehr D. Tissue electrolyte alteration in disseminated
myocardial necrosis. Ann NY Acad Sci 1969;156:344-378.
15. Schroeder HA. Relations between mortality from
cardiovascular disease and treated water supplies. JAMA
1960:172:1902-1908.
16. Karppanen H, Neuvoenen PJ. Ischemic heart disease and soil
magnesium in Finland (letter). Lancet 1973;2.1390.
17. Marier JR. Role of magnesium in the "hard water story".
Magnesium Bull 1986;8:194-198.
18. Anderson T, Neri LC, Schreiber GB, Talbot F, Zdrowjewski
A. Ischemic heart disease, water hardness, and myocardial
magnesium. Can Med Assoc J 1975;113:199-203.
19. Anderson TW, LeRiche WH: Sudden death from ischemic heart
disease in Ontario and its correlation with water hardness and
other factors. Can Med Assoc J 1971;105:155-160.
20. Altura BM, Altura BT, Carella A, Prasad DMV.
Hypomagnesemia and vasoconstriction: possible relationship to
etiology of sudden death ischemic disease and hypertensive
vascular disease. Artery 1981;9:212-213.
21. Whang R, Flink EB, Dyckner T, Wester PO, Aikawa JK, Ryan
MP. Magnesium depletion as a cause of refractory potassium
repletion. Arch Intern Med 1985;145:1686-1689.
22. Ryan MP: Diuretics and potassium/magnesium depletion.
Directions for treatment. Am J Med 1987;82(Suppl 3A):38-47.
23. Dyckner T, Wester PO. Potassium/magnesium depletion in
patients with cardiovascular disease. Am J Med 1087;82(Suppl
3A):11-17.
24. Cohen JJ, Kassirer JP. Acid base metabolism. In Maxwell
MH, Kleeman CR, eds. Clinical Disorders of Fluid and Electrolyte
Metabolism. New York:McGraw-Hill Book Co, 1980:1812-232.
25. Field MJ, Lawrence JR. Complications of thiazide therapy:
an update. Med J Aust 1986;144:641-644.
26. Seelig MS: The requirement of magnesium by the normal
adult. Am J Clin Nutr 1964;14:342-390.
27. Seelig MS: Magnesium requirements in human nutrition.
Magnesium Bull 1981;3 (Suppl 1A):26-47.
28. Committee on Dietary Allowance: Recommended dietary
allowances. Magnesium. 9th Revised Edition. National Academy of
Sciences, Washington, D.C., 1980, pp 134-136.
29. USDA. Food and nutrient intakes of individuals on 1 day in
the U.S., Spring 1977. Nationwide Food Consumption Survey
1977-1978. Preliminary Report #2, Washington DC, 1980.
30. Morgan KJ, Stampley GL, Zabik ME, Fischer DR. Magnesium
and calcium intakes of the U.S. population. J Am Coll Nutr
1985;4: 195-206.
31. Lakshmanan FL, Rao RB, Kim WW, Kelsay JL. Magnesium
intakes, balances, and blood levels of adults consuming
self-selected diets. Am J Clin Nutr 1984;40:1380-1389.
32. Walker M, Page L. Nutritive content of college meals. III.
Mineral elements. J Am Diet Assoc 1977;70:260-266.
33. Srivastava US, Nadeau MJ, Guennou L. Nutrition Reports
Intl 1978;18:235-242.
34. White HS. Inorganic elements in weighed diets of girls and
young women. J Am Diet Assoc 1969;55:38-43.
35. Seelig MS, Haddy FJ. Magnesium and the arteries. I.
Effects of magnesium deficiency on arteries and on the retention
of sodium, potassium and calcium. in Cantin M, Seelig MS, eds.
Magnesium in Health and Disease. New York: SP Med & Sc Books,
1980:605-638.
36. Rayssiguier Y. Magnesium and lipid interrelationships in
the pathogenesis of vascular diseases. Magnesium Bull
1981;3:165-177.
37. Rayssiguier Y. Lipoprotein metabolism: importance of
magnesium. Magnesium Bull 1986;8:186-193.
38. Rayssiguier Y, Gueux E. Magnesium and lipids in
cardiovascular disease. J Am Coll Nutr 1986;5:507-519.
39. Vitale JJ, White PL, Nakamura M, Zamcheck N, Hellerstein
EE. Interrelationships between experimental hypercholesterolemia,
magnesium requirements, and experimental atherosclerosis. J Exp
Med 1957;106:757-766.
40. Hellerstein E, Vitale JJ,White PL, Hegsted D, Zamcheck N,
Nakamura M. Influence of dietary magnesium on cardiac and renal
lesions of young rats Fed an atherogenic diet. J Exp Med 1957:
106:767-775.
41. Vitale JJ, Velez H, Guzman C, Correa P. Magnesium
deficiency in the cebus monkey. Circ Res 1963;12:642-650.
42. Elin RJ: Magnesium Metabolism in Health and Disease.
Disease-a- Month 34: 161-218, 1988.
43. Szelenyi Y, Rigo R, Ahmen BO, Sos J. The role of magnesium
in blood coagulation. Thromb Diath Haemaorrh 1967;18:626-633.
44. Rayssiguier Y, Gueux E, Weiser D. Effect of magnesium
deficiency on lipid metabolism in rats fed a high carbohydrate
diet. J Nutr 1981;111:1876-1883.
45. Rayssiguier Y, Gueux E. The reduction of plasma
triglyceride clearance by magnesium-deficient rats. Magnesium
1983;2:132-138.
46. Kruse H, Orent E, McCollum E. Studies on magnesium
deficiency in animals. III. Chemical Changes in the blood
following magnesium deprivation. J Biol Chem
1933;100:603-643.
47. Gueux E, Rayssiguier Y, Piot M-C, Alcindor L: Reduction of
plasma lecithin-cholesterol acyl-transferase activity by acute
magnesium deficiency in the rat. J Nutr 1984;114:1479-1483.
48. Davis WH, Leary WP, Reyes AJ, Ohlaberry JV: Monotherapy
with magnesium increases abnormally low high density lipoprotein
cholesterol: a clinical assay. Curr Ther Res 36;1984:341-344.
49. Lindeman RD, Adler S, Yiengst MJ, Beard ES. Influence of
various nutrients on urinary divalent cation excretion. L Lab
Clin Med 1967;70:236-245.
50. Martin HE.: Clinical magnesium deficiency. Ann NY Acad Sci
1969;156:891-900.
51. Martin HE, Wertman M. Serum potassium, magnesium and
calcium levels in diabetic acidosis. J Clin Invest
1947;26:217-228.
52. Ditzel J. Morphologic and hemodynamic changes in the
smaller blood vessels in diabetes mellitus. I. Considerations
based on the literature. New Engl J Med 1954;250:551-546.
53. James TN. Pathology of small coronary arteries. Am J
Cardiol 1967;20:679-691.
54. Linden V. Correlation of vitamin D intake to ischemic
heart disease, hypercholesterolemia, and renal calcinosis. In
Seelig MS ed. Nutritional Imbalances in Infant and Adult Disease:
Mineral, Vitamin D and Cholesterol. New York, Spectrum Pub 1977:
23-42.
55. Linden V, Seelig MS. Multiple factors in the
hyperlipidemia of hypervitaminosis D (letter). Br Med J
1975;4:166.
56. Harris LJ. The mode of action of vitamin D. The
"parathyroid" theory: clinical hypervitaminosis. Lancet
1932;1:1031-1038.
57. Forfar JO, Tompsett SL, Forshall W. Biochemical studies in
idiopathic hypercalcemia in infancy. Arch Dis Childh 1959;34:
525-537.
58. Seelig MS. Vitamin D and cardiovascular, renal, and brain
damage in infancy and childhood. An NY Acad Sci 1969;147:
537-582. 59. Seelig MS. Magnesium deficiency with
phosphate and vitamin D excesses: role in pediatric
cardiovascular disease? Cardiovasc Med 1978;3:637-650.
60. Norman AW, Henry H. 1,25-dihydroxycholecalciferol - a
hormonally active form of vitamin D3. Rec Prog Horm Res
1974;30:431-430. 61. Sos J. An investigation into nutritional
factors of experimental cardiopathy. In Bajusz E & Rona G,
eds.Electrolytes and Cardiovascular Disease: Fundamental Aspects.
Baltimore: Williams & Wilkins, 1965:161-180.
62. Selye H. The Pluricausal Cardiomyopathies. Springfield IL:
Charles C Thomas, 1961.
63. Karppanen H. Epidemiologic studies on the relationship
between magnesium intake and cardiovascular diseases. Artery
1981;9: 190-199.
64. Kalbfleisch JM, Lindeman RD, Ginn HE, Smith WO. Effects of
ethanol administration on urinary excretion of magnesium and
other electrolytes in alcoholic and normal subjects. J Clin
Invest 1963;42:1471-1475.
65. McCollister RJ, Prasad AS, Doe RP, Flink EB. Normal renal
magnesium clearance and the effect of water loading,
chlorthiazide and ethanol on magnesium clearance. J Lab Clin Med
1958;52:928.
66. McCollister RJ, Flink EB, Lewis MD. Urinary excretion of
magnesium in man following the ingestion of alcohol. Am J Clin
Nutr 1963;12:415-420.
67. Flink EB, Stutzman FL, Anderson AR, Konig T, Fraser R:
Magnesium deficiency after prolonged parenteral fluid
administration and after chronic alcoholism complicated by
delirium tremens. J Lab Clin Med 1954;43:956-968.
68. Flink EB: Magnesium deficiency in alcoholism. Clin Exp Res
1986; 10:590-594, .
69. Horton R, Biglieri EG. Effect of aldosterone on the
metabolism of magnesium. J Clin Endocrin Metab
1962;22:1187-1192.
70. Flink EB, Shane SR, Scobbo RR, Blehschmidt NG, McDowell P.
Relationship of free fatty acids and magnesium in ethanol
withdrawal in Dogs. Metabolism 1979;28:858-865.
71. Zieve L. Influence of magnesium deficiency on the
utilization of thiamine. Ann NY Acad Sci 1969;162:732-743.
72. Itokawa Y, Tseng L-F, Fujiwara M. Thiamine metabolism in
magnesium-deficient rats. J Nutr Sc Vit 1974;20:249-255.
73. Itokawa Y, Inoue K, Natori Y, Okazaki K, Fujiwara M.
Effect of thiamine on growth, tissue magnesium and thiamine
levels and on transketolase in magnesium deficient rats. J
Vitaminol 1972;18: 159-164.
74. Seelig MS. Nutritional status and requirements of
magnesium. Magnesium Bull 1986;8:170-185.
75. Hoffstrom KA: Phosphor, Calcium and Magnesium. Skand Arch
Physiol 1916;23:326-420.
76. Franz KB: Magnesium intake during pregnancy. Magnesium
1985;6: 18-27.
77. Endres JM, Sawicki M, Casper JA. Dietary assessment of
pregnant women in a supplemental food program. J Am Diet Assoc
1981;79: 121-126.
78. Ashe JR, Schofield FA, Gram MR. The retention of calcium,
phosphorus and magnesium during pregnancy: the adequacy of
prenatal diets with and without supplementation. Am J Clin Nutr
1979;32:286-291.
79. Johnson NE, Phillips CA: Magnesium contents of pregnant
women. In Cantin M, Seelig MS, eds. Magnesium in Health &
Disease, New York: SP Med & Sci Books 1980: 827-831.
80. Seelig MS: Possible roles of magnesium in disorders of the
aged. In Regelson W, Sinex FM, eds. Intervention in the Aging
Process, Part A: Quantitation, Epidemiology, Clinical Research.
New York: AR Liss, Inc, 1983:279-205.
81. Johansson G. Magnesium metabolism. Studies in health,
primary hyperparathyroidism and renal stone disease. Scand J Urol
Nephrol 1979;51:1-47.
82. Mountakalakis TD. Effects of aging, chronic disease, and
multiple supplements on magnesium requirements. Magnesium
1987:6:5-11.
83. Vir SC, Love AHG. Nutritional status of institutionalized
and non-institutionalized Aged in Belfast. Am J Clin Nutr
1979;32: 1934-1947.
84. Fehlinger R, Fauk D, Seidel K. Hypomagnesemia and
transient ischemic cerebral attacks. Magnesium Bull
1984;6:100-104.
85. Heroux O, Peter D, Heggtveit A. Long-term effect of
subnormal magnesium on magnesium and calcium contents of organs,
on cold tolerance and on lifespan and its pathological
consequences in rats. J Nutr 1977;107:1640-1652.
86. Cantin M. Relationship of juxtaglomerular apparatus and
adrenal cortex to biochemical and extracellular fluid volume
changes in magnesium deficiency. Lab Investig
1970;22:558-568.
87. Mader IJ, Iseri LT. Spontaneous hypopotassemia,
hypomagnesemia, alkalosis and tetany due to hypersecretion of
corticosterone- like mineralocorticoid. Am J Med
1955;19:976-988.
88. Douglas WW, Rubin RP. The Effects of alkaline earths and
other divalent cations on adrenal medullary secretion. J Physiol
1964;175:231-241.
89. Boullin D.J. The action of extracellular cations on the
release of the sympathetic transmitter from peripheral nerves. J
Physiol 1967;89:85-99.
90. Ising H. Interaction of noise-induced stress and Mg
decrease. Artery 1981;9:205-211.
91. Ising H, Bertschat F, Ibe K, Stoboy V, Goossen C, Hengst
G. Stress-induced Ca/Mg shifts and vascular response in animals
and men; comparison to electrolyte alterations in myocardial
infarction patients. Magnesium Bull 1986;8:95-103.
92. Ebel H, Guenther T: Role of magnesium in cardiac disease.
J Clin Chem Clin Biochem 1983; 21:249-256.
93. Lehr D, Krukowski M, Colon R. Correlation of myocardial
and renal necrosis with tissue electrolyte changes. JAMA 1966;
197:105-112.
94. Kraikitpanitch S, Haygood CC, Baxter DJ, Yunice AA,
Lindeman RD. Effects of acetylsalicylic acid, dipyridamole, and
hydrocortisone on epinephrine-induced myocardial injury in dogs.
Am Heart J 1976;92:615-622.
95. Lehr D. Magnesium and cardiac necrosis. Magnesium Bull
1981; 3(1A):178-191.
96. Abraham AS, Baron E, Eylath U. Changes in the magnesium
content of tissues following myocardial damage in rats. Med Biol
1981;59: 99-102.
97. Fleckenstein A, Janke J, Doering HJ, Pachinger O.
Myocardial fiber necrosis due to intracellular Ca overload - a
new principle in cardiac pathophysiology. Rec Adv Stud Card
Struct Metabol 1972;4:563-580.
98. Spah F, Fleckenstein A. Evidence of a new preferentially
Mg- carrying transport system besides the fast Na and the slow Ca
channels in the excited myocardial sarcolemma membrane. J Mol
Cell Cardiol 1979;11:1109-1129.
99. Maguire ME, Erdos JJ. Magnesium but not calcium
accumulation is inhibited by B-adrenergic stimulation in S49
lymphoma cells. J Biol Chem 1978;253:6633-6635.
100. Rayssiguier Y. Hypomagnesemia resulting from adrenalin
infusion in ewes: its relation to lipolysis. Horm Metab Res
1977;9:309-314.
101. Flink EB, Brick JE, Shane SR: Alterations of long-chain
free fatty acid and magnesium concentrations in acute myocardial
infarction. Arch Intern Med 1981;141:441-443.
102. Elliott DA, Rizak MA. Epinephrine and adrenocorticotropic
hormone-stimulated magnesium accumulation in adipocytes and their
plasma membranes. J Biol Chem 1973;249:3985-3990.
103. Vormann J, Foerster R, Guenther T, Ebel H.
Lipolysis-induced Mg uptake into fat cells. Magnesium Bull
1983;5:39-41.
104. Altura BT. Type A behavior and coronary vasospasm: a
possible role of hypomagnesemia. Med Hypoth 1980;6:753-757.
105. Henrotte JG. Type A behavior and magnesium metabolism.
Magnesium
106. Simon J, Kruzej E, Svarc V, Krizk M. Correlation of A B
behavior pattern, alcohol intake, HDL-cholesterol and serum
magnesium levels in middle-aged men. Act Nerv Super
1983;25:105-107.
107. Henrotte JG, Plouin PF, Levy-Leboyer G, Moser G,
Sideroff- Girault N, Franck G, Santarromana M, Pineau M. Blood
and urinary magnesium, zinc, calcium, free fatty acids and
catecholamines in type A and type B subjects. J Am Coll Nutr
1985;4:165-172.
108. Durlach J (Transl by D Wilson). Magnesium in Clinical
Practice. London: J Libbey & Co, 1988.
109. Classen H-G, Classen O, Fischer G, Fischer H, Helbig J,
Rummler HG, Schimatschek H. Magnesium: Prevention of
stress-induced cardiovascular damage. Magnesium Bull
1986;8:140-144.
110. Ducroux T: L'Enfant spasmophile. Aspects diagnostiques et
therapeutiques. Magnesium Bull 1984;6:9-16.
111. Heggtveit HA. The cardiomyopathy of Mg-deficiency. In
Bajusz E, ed. Electrolytes and Cardiovascular Diseases, Vol 1.
New York: S Karger, 1965:204-220.
112. Heggtveit HA, Tanser L, Mishra RK. Cardiac necrosis and
calcification in experimental magnesium deficiency. Am J Path
1964;45:757-782.
113. Sordahl LA. Effects of magnesium, ruthenium red, and the
antibiotic ionophore A-23187 on initial rates of calcium uptake
and release by heart mitochondria. Arch Biochem Biophys
1974;167:103-115.
114. Sordahl LA, Silver BB. Pathological accumulation of
calcium by mitochondria: modulation by magnesium. Rec Adv
Structure & Metabolism 1975;6:85-93.
115. Janke J, Fleckenstein A, Hein B, Leder O, Sigel H.
Prevention of myocardial Ca overload and necrotization by Mg and
K salts or acidosis. Rec Adv Stud Card Struct Metab
1975;6:33-42.
116. Lehr D, Chau R, Irene S. Possible role of magnesium loss
in the pathogenesis of myocardial fiber necrosis. Rec Adv Stud
Card Struct Metab 1975;6:258-273.
117. Heggtveit HA. Contribution of electron microscopy to the
study of myocardial ischemia. WHO 1971;41:865-872.
118. Altura BM, Altura BT. New perspectives on the role of
magnesium in the pathophysiology of the cardiovascular system, I.
Clinical aspects. Magnesium 1985;4:226-244.
119. Altura BM, Altura BT. New perspectives on the role of
magnesium in the pathophysiology of the cardiovascular system.
II. Experimental aspects. Magnesium 1985;245-271.
120. Bloom S. Coronary arterial lesions in Mg-deficient
hamsters. Magnesium 1985:4:82-95.
121. Duguid JB. Thrombosis as a factor in the pathogenesis of
coronary atherosclerosis. J Pathol Bacteriol 1946;58:207-212.
122. Stevenson MM, Yoder I. Studies of platelet aggregation,
plasma adenosine diphosphate breakdown, and blood coagulation in
magnesium deficient calves and rats. Thromb Diath Hemorrh 1972;
23:299-305.
123. Adams JH, Mitchell JRA: The effect of agents which modify
platelet behavior and of magnesium ions on thrombus formation in
vivo. Thrombos Haemostas 1979;42:603-610.
124. Gertz SD, Wajnberg RS, Kurgan A, Uretzky G. Effect of
magnesium sulfate on thrombus formation following partial
arterial constriction: implications for coronary vasospasm.
Magnesium 1987;6:225-235.
125. Durlach J. Magnesium deficiency thrombosis (letter).
Lancet 1967;1:1967.
126. Durlach J. Pilule, et thrombose (des plaquettes, des
estrogens et du magnesium). Rev Fr Endocrinol Clin
1970;11:45-54.
127. Hughes A Tonks RS. Platelets, magnesium and myocardial
infarction. Lancet 1965;1:1044-1046.
128. Cohen L, Kitzes R. Prompt termination and/or prevention
of coldpressor-stimulation-induced vasoconstriction of different
beds by magnesium sulfate in patients with Prinzmetal's angina.
Magnesium 1986;5:144-149.
129. Roberts WC, Buja LM. The frequency and significance of
coronary arterial thrombi and other observations in fatal acute
myocardial infarction. Am J Med 1972;52:425-443.
130. Hudson REB. The cardiomyopathies: order from chaos. Am J
Cardiol 1970;25:70-77.
131. Altura BM, Ischemic heartdisease and magnesium. Magnesium
1988; 7:57-67.
132. Ross R, Glomsett JA. The pathogenesis of atherosclerosis.
N Engl L Med 1976;195:420-425.
133. Borchgrevink PC, Oksendal AN, Jynge P. Acute
extracellular magnesium deficiency and myocardial tolerance to
ischemia. J Am Coll Nutr 1987;6:125-130.
134. Chang C, Varghese PJ, Downey J, Bloom S. Magnesium
deficiency and myocardial infarct size in the dog. J Am Coll
Cardiol 1985;5: 280-289.
135. Hearse DJ, Stewart DA, Braimbridge MVL. Myocardial
protection during ischemic cardiac arrest. The importance of
magnesium in cardioplegic infusates. J Thor Cardiavasc Surg
1978;75:877-885.
136. Shattock MJ, Hearse DJ, Fry CH: The ionic basis of the
anti- ischemic and anti-arrhythmic properties of magnesium in the
heart. J Am Coll Nutr 1987;6:27-33.
137. Mansford KRL, Hearse DJ. Metabolic approaches to
myocardial infarction. In Hems DA, ed.Biologically Active
Substances: Exploration and Exploitation. Chichester: John Wiley
& Sons, 1977:239-264.
138. Greenberg D, Tufts E. The nature of magnesium tetany. Am
J Physiol 1938;121:416-423.
139. Syllm-Rapoport I, Strassburger I, Gruneberg D, Zirbel C.
Electrocardiographic studies in dogs with experimental magnesium
deficiency. J Paediat 1962; 60:801-804.
140. Wener J, Pintar K, Simon MA, Motola R, Friedman R, Mayman
A, Schucher R. The effects of prolonged hypomagnesemia on the
cardiovascular system in young dogs. Am Heart J
1964;67:221-231.
141. Vitale JJ, Hellerstein EE, Nakamura M, Lown B. Effects of
magnesium-deficient diet upon puppies. Circ Res
1961;9:387-394.
142. Seelig MS. Electrocardiographic patterns of magnesium
depletion appearing in alcoholic heart disease. Ann NY Acad Sci
1969; 162:906-917.
143. Burch GE, Giles TD. The importance of magnesium
deficiency in cardiovascular disease. Am Heart J
1977;4:649-657.
144. Sheehan JP, Seelig MS. Interactions of magnesium and
potassium in the pathogenesis of cardiovascular disease.
Magnesium 1984;301- 314.
145. Rude RK, Oldham SB, Sharp CF, Singer FR. Parathyroid
hormone secretion in magnesium deficiency. J Clin Endocrinol
Metab 1978; 47:800-806.
146. Iseri LT, French JH. Magnesium: nature's physiologic
calcium blocker. Am Heart J 1984;108:188-193.
147. Dyckner TH, Wester PO. Magnesium-electrophysiological
effects. Magnesium Bull 1986;8:219-222.
148. Kulick DL, Hong R, Ryzen E, Rude RK, Rubin JN, Elkayam U,
Rahimtoola SH, Bhandari AK. Electrophysiologic effects of
intravenous magnesium in patients with normal conduction systems
and no clinical evidence of significant cardiac disease. Am Heart
J 1988;115:367-373.
149. Abraham AS, Rosenman D, Meshulam Z, Zion M, Eylath U.
Serum, lymphocyte, and erythrocyte potassium, magnesium, and
calcium concentrations and their relation to tachyarrhythmias in
patients with acute myocardial infarction. Am J Med
1986;81:983-988.
150. Ising H, Guenther T, Bertschat F, Ibe K, Stoboy V,
Heldman E. Alterations of electrolytes in serum and erythrocytes
after myocardial infarction. Magnesium 1987;6:192-200.
151. Abraham AS. Potassium and magnesium status in ischaemic
heart disease. Magn Res 1988;1: 53-57.
152. Rasmussen HS, McNair P, Goransson L, Balslov S, Larsen
OG, Aurup P. Magnesium deficiency in patients with ischemic heart
disease with and without acute myocardial infarction uncovered by
an intravenous loading test. Arch Intern Med
1988;148:329-332.
153. Ryzen E, Elkayam U, Rude RK. Low blood mononuclear cell
magnesium in intensive cardiac care unit patients. Am Heart J
1986;111: 475-480.
154. Randall RE Jr, Rossmeisl EC, Bleifer KH. Magnesium
depletion in man. Ann Intern Med 1959;50:257-287.
155. Brigden W, Robinson J. Alcoholic heart disease. Br Med J
1964; 2:1283-1289.
156. Fankushen D, Raskin D, Dimich A, Wallach S. The
significance of hypomagnesemia in alcoholic patients. Am J Med
1964;37:802-812.
157. Loeb HS, Raymond P, Gunnar RM, Tobin JR. Paroxysmal
ventricular fibrillation in two patients with hypomagnesemia.
Treatment by transvenous pacing. Circulation 1968;37:210-215.
158. Ricketts HH, Denison EK, Haywood IJ. Unusual T-wave
abnormality. Repolarization alternans associated with
hypomagnesemia, acute alcoholism and cardiomyopathy. JAMA
1969;207:365-366.
159. Luomanmaki K, Heikkila J, Hartikainen M. T-wave alternans
associated with heart failure and hypomagnesemia in alcoholic
cardiomyopathy. Europ J Cardiol 1975;3:167-170.\
160. Kellaway G, Ewen K. Magnesium deficiency complicating
prolonged gastric suction. New Zeal Med J 1962;61:137-142.
161. Thoren L. Magnesium deficiency in gastrointestinal fluid
loss. Acta Chir Scand 1963;Suppl 306:1-65.
162. Levine SR, Crowley TJ, Hai HA. Hypomagnesemia and
ventricular tachycardia. Chest 1982;81:244-247.
163. Lim P, Jacob E. Tissue magnesium level in chronic
diarrhea. J Lab Clin Med 1972;80:313-321.
164. Caddell JL. The effect of magnesium therapy on
cardiovascular and electrocardiographic changes in severe
protein-calorie malnutrition. Trop Geogr Med 1969;21:33-38.
165. Dooling ED, Stern L. Hypomagnesemia with convulsions in a
newborn infant. Report of a case associated with maternal
hypophosphatemia. Can Med Assc J 1967;97:827-831.
166. Bajpai PC, Hasan M, Gupta AK, Kunwar KB.
Electrocardiographic changes in hypomagnesemia. Indian Heart J
1972;24:271-276.
167. Killen DA, Grogan EL II, Gower IE, Collins IS, Collins
HA. Effect of ACD blood prime on plasma calcium and magnesium.
Ann Thorac Surg 1972;18:371-380.
168. Scheinman MM, Sullivan RW, Hyatt KH. Magnesium metabolism
in patients undergoing cardiopulmonary bypass. Circulation
1969;39- 40(Suppl I);40:1235-1241.
169. Holden MP, Ionescu MI, Wooler GH. Magnesium in patients
undergoing open-heart surgery. Thorax 1972;27:212-218.
170. Holden MP. The value of magnesium supplements during
open-heart surgery. A double-blind trial. In Naito KH, ed.
Nutrition and Heart Disease. SP Press 1982:273-283.
171. Khan RMA, Hodge JS, Bassett HFM. Magnesium in open-heart
surgery. J Thorac Cardiovasc Surg 1973;66:185-191.
172. Hearse DJ, Stewart DA, Braimbridge MV. Cellular
protection during myocardial ischemia. The development and
characterization of a procedure for the induction of reversible
ischemic arrest. Circulation 1976;54:193-202.
173. Morton BC, Smith FM, McKibbon TJ, Nair RC, Poznanski WJ.
Magnesium therapy in acute myocardial infarction. Magnesium Bull
1981;3(1A):192-194.
174. Morton BC, Smith FM, Nair RC, McKibbon TG, Poznanski WJ.
The clinical effects of magnesium sulphate treatment in acute
myocardial infarction. Magnesium Bull 1984;4:133-136.
175. Rasmussen HS, McNair P, Norregard P, Backer V, Lindeneg
O, Balslev S. Intravenous magnesium in acute myocardial
infarction. Lancet 1986:234-236.
176. Abraham AS, Rosenman D, Kramer M, Balkin J, Zion MM,
Farbstein H, Eylath U. Magnesium in the prevention of lethal
arrhythmias in acute myocardial infarction. Arch Intern Med
1987;147:753-755.
177. Rasmussen HS. Justification for intravenous magnesium
therapy in acute myocardial infarction. Magn Res
1988;1:59-73.
178. Dyckner T. Serum magnesium in acute myocardial
infarction. Acta med scand 1980;207:59-66.
179. Packer M. Sudden unexpected death in patients with
congestive heart failure: a second frontier. Circulation
1985;72:681-685.
180. Wester PO, Dyckner T. Intracellular electrolytes in
cardiac failure. Acta Med Scand 1986;707Suppl:33-36.
181. Wester PO, Dyckner T. Magnesium in cardiac failure and
diuretic treatment. Magnesium Bull 1986;8:204-209.
182. Packer M, Gottlieb SS, Blum MA. Immediate and long-term
pathophysiologic mechanisms underlying the genesis of sudden
cardiac death in patients with congestive heart failure. Am J Med
1987;82 (Suppl 3A):4-10, 1987.
183. Francis GS. Neurohumoral mechanisms involved in
congestive heart failure. Am J Cardiol 1985;55:15A-21A.
184. Dyckner T, Wester PO: Ventricular extrasystoles and
intracellular electrolytes before and after potassium and
magnesium infusions in patients on diuretic treatment. Am Heart J
1979;97:12-18.
185. Luccioni R, Frances Y, Kiegel P, Collet F. Prolapsus
valvulaire mitral - spasmophilie et deficit magnesien. Magnesium
Bull 1982;4:62-67.
186. Galland LD, Baker SM, McLellan RK. Magnesium deficiency
in the pathogenesis of mitral valve prolapse. Magnesium
1986;5:165-174.
187. Henrotte JG. The variability of human red blood cell
magnesium level according to HLA groups. Tissue-Antigens
1980;15:419-430.
188. Braun WE, Ronan JA, Schachter B, Gardin J, Isner J, Greek
D. HLA antigens in mitral valve prolapse. Transplant Proc
1977;9:1869- 1871.
189. Kaplan NM: Dietary aspects of the treatment of
hypertension. Ann Rev Public Health 1986;7:503-519.
190. McCarron DA, Morris CD, Cole C. Dietary calcium in human
hypertension. Science 1982;267-269, .
191. Kaplan NM, Meese RB. The calcium deficiency hypothesis of
hypertension: a critique. Ann Intern Med 1986;105:947-955.
192. Haddy FJ, Seelig MS. Magnesium and the Arteries. II.
Physiologic effects of electrolyte abnormalities on arterial
resistance. In Cantin M, Seelig MS, eds. Magnesium in Health and
Disease. New York: SP Med & Sc Books, 1980:639-657.
193. Altura BM. Altura BT. Interactions of Mg and K on blood
vessels - aspects in view of hypertension. Review of present
status and new findings. Magnesium 1984;3:175-194.
194. Altura BM, Altura BT. Role of magnesium ions in
contractility of blood vessels ans skeletal muscles. Magn
Bulletin 1981;3:102-114.
195. Seelig MS, Berger AR, Avioli LA. Speculations on renal
hormonal, and metabolic aberrations in a patient with marginal
magnesium deficiency. In Cantin M, MS Seelig, eds. Magnesium in
Health and Disease. New York: SP Med & Sc Books,
1980:459-468.
196. Resnick LM, Laragh JH, Sealy JE, Alderman MH. Divalent
cations in essential hypertension. New Engl J Med
1983;309:888-891.
197. Resnick LM, Gupta RK, Laragh JH. Intracellular free
magnesium in erythrocytes of essential hypertension: relation to
blood pressure and serum divalent cations. Proc Natk Acad Sci USA
1984;81:6511-6515.
198. Resnick LM. Uniformity and diversity of calcium
metabolism in hypertension. A conceptual framework. Am J Med
1987;82(suppl 1B): 16-26.
199. Dyckner T, Wester PO. Effects of magnesium infusions in
diuretic induced hyponatremia. Lancet 1981;1:585-586.
200. Dyckner T, Wester PO. Effect of magnesium on blood
pressure. Br Med J 1983;286:1847-1849.
201. Zwillinger L. On magnesium's effect on the heart. Klin
Wochenschr 1935;14:1429-1433. (in German)
202. Kim YW, Andrews CE, Ruth WE. Serum magnesium and cardiac
arrhythmias with special reference to digitalis intoxication. Am
J Med Sci 1961;242:87-92.
203. Beller GA, Hood WB Jr, Smith TW, Abelmann WH, Wacker WEC.
Correlation of serum magnesium levels and cardiac digitalis
intoxication. Am J Cardiol 1974;33:225-229.
204. Iseri LT. Potassium, magnesium, and digitalis toxicity.
In Whang R, ed. Potassium: Its Biologic Significance . Boca Raton
FLa: CRC Press, 1983:125-135 .
205. Cohen L, Kitzes R. Magnesium sulfate and digitalis toxic
arrhythmias. JAMA 1983;249:2808-2810.
206. Whang R, Oei TO, Watanabe A. Frequency of hypomagnesemia
in hospitalized patients receiving digitalis. Arch Intern Med
1985; 145:655-656.
207. DeCarli C, Sprouse G, LaRosa JC. Serum magnesium levels
in symptomatic atrial fibrillation and their relation to rhythm
control by intravenous digoxin. Am J Cardiol 1986;57:956-959.
208. Kass RS, Lederer WJ, Tsien RW, Weingart R. Role of
calcium ions in transient inward current and after contraction
induced by strophanthidin in cardiac Purkinje fibers. J Physiol
(London) 1978;281:187-208.
209. Specter MJ, Schweizer E, Goldman RH. Studies on
magnesium's mechanism of action in digitalis induced arrhythmias.
Circulation 1975;52:1001-1005.
210. Ghani MF, Smith JR. The effectiveness of magnesium
chloride in the treatment of ventricular tachyarrhythmias due to
digitalis intoxication. Am Heart J 1974;88:621-626.
211. MacIntyre I, Hanna S, Booth CC, Read AE. Intracellular
magnesium deficiency in man. Clin Sci 1961;20:297-305.
212. Lim P, Jacob E, Dong S, Khoo OT. Values for tissue
magnesium as a guide for detecting magnesium deficiency. J Clin
Path 1969; 22:417-421.
213. Merrill JP, Hampers CL. Uremia (Med Progr Series). N Engl
J Med 1970;282:1014-1021.
214. Sheehan J, White A. Diuretic-associated hypomagnaesemia.
Br Med J 1982;285:1157-1159.
215. Martin BJ, Milligan K: Diuretic-associated hypomagnesemia
in the elderly. Arch Intern Med 1987;1768-1771.
216. Hollifield JW. Magnesium depletion, diuretics, and
arrhythmias. Am J Med 1987;82 (Suppl 3A):30-37.
217. Multiple Risk Factor Intervention Trial Research Group.
Multiple risk factor intervention trial: risk factor changes and
mortality results. JAMA 1982;248:2465-2477.
218. Sherwin R. Sudden death in men with increased risk of
myocardial infarction - the MRFIT Program. Drugs 1984;28 (Suppl
1):46-53.
219. Whelton PK. Diuretics and arrhythmias in the Medical
Research Council Trial. Drugs 1984;28 (Suppl 1):54-65.
220. Morgan TO, Adams WR, Hodgson M, Gibberd RW. Failure of
therapy to improve the prognosis in elderly males with
hypertension. Med J Aust 2: 27-31, 1980.
221. Amery A, Birkhager W, Brixko P.: Mortality and morbidity
results from the European Working Party on high blood pressure in
the elderly. Lancet 1985;1:1249-1354.
222. Kuller L, Farrier N, Caggiula A, Borhani N, Dunkle S.
Relationship of diuretic therapy and serum magnesium levels among
participants in the Multiple Risk Facor Intervention Trial. Am J
Epidemiol 1985;122:1045-1059.
223. Holliday MA, Lukenbill A, Hancock C. Acute metabolic
alkalosis: its effect on potassium and acid excretion. J Clin
Invest 1955; 34:428-433.
224. Atkins EL, Schwartz WB: Factors governing correction of
the alkalosis associated with potassium deficiency; the critical
role of chloride in the recovery process. J Clin Invest 1962;41:
218-229.
225. Kassirer JP, Berkman PM, Lawrenz DR, Schwartz WB. The
critical role of chloride in the correction of hypokalemic
alkalosis in man. Am J Med 1965;38:172-190.
226. Iseri LT, Fairshter RD, Hardemann JL, Brodsky MA.
Magnesium and potassium therapy in multifocal atrial tachycardia.
Am Heart J 1985;110:789-794.
227. Whang R, Wagner R. The influence of venous occlusion and
exercise on serum magnesium concentration. Metabolism
1966;15:608-612.
228. Engel RR, Elin RJ. Hypermagnesemia from birth asphyxia. J
Pediat 1970;77:631-637.
229. Brater DC, Morrelli HF. Systemic alkalosis and digitalis
related arrhythmias. Acta Med Scand 1981;647:79-85.
230. Urakabe S, Nakata K, Ando A, Orita Y, Abe H. Hypokalemia
and metabolic alkalosis resulting from overuse of magnesium
oxide. Jpn Circ J 1975;39:1135-113.
231. Schimatschek HT, Classen HG, Thoni H, Haubold W.
Acid-base changes in rats kept on highly magnesium enriched diets
using different magnesium compounds. Magn Bulletin
1987;9:161-176. (in German)
232. Classen H-G, Marquardt P, Spaeth M, Ebel H, Schumacher
K-A. Improvement by chlorine of the intestinal absorption of
inorganic and organic Mg-compounds and their protective effect
against adrenergic cardiopathy. Rec Adv Stud Card Struct Metab
1975;111-119.
233. Classen H-G. Magnesium and potassium deprivation and
supplementation in animals and man: aspects in view of intestinal
absorption. Magnesium 1984;2:257-264.
234. Classen H-G, Fischer G, Jacob R, Marx J,Schimatschek H,
Stein C. Prenecrotic electrolyte alterations of the adrenergic
cardiopathy: potentiation by magnesium depletion and prevention
by high dietary magnesium levels and verapamil. Magnesium
Bulletin 1986;8: 82-92.
235. Bajusz E. Age factor and myocardial necrosis:
experimental studies on the sensitizing effect of K-, Mg-, and
Cl- deficiencies. Gerontologia 1963;8:66-80.
236. Davis WH, Ziady F. The effect of oral magnesium chloride
therapy on the QTc and QTu intervals of the electrocardiogram. S
Afr Med J 1978;53:591-593.
237. Reyes AJ, Leary WP, Acosta-Barrios TN, Davis WH.
Magnesium supplementation in hypertension treated with
hydrochlorthiazide. Curr Ther Res 36:332-340, 1984.
This page was first uploaded to The Magnesium Web Site on
October 18, 1995
http://www.mgwater.com/