ORIGINAL ARTICLE
Magnesium Research (1997) 10, 2,
169-195
Review paper
Neurotic, neuromuscular and autonomic nervous form of
magnesium imbalance
Jean Durlach*, Pierre Bac†, Vincent Durlach‡,
Michel Bara § and André Guiet-Bara
*SDRM, Hôpital Saint-Vincent-de-Paul, 74-82 Avenue,
Denfert-Rochereau, F-75014 Paris, France; †Laboratoire de
Pharmacologie, Faculté - de Pharmacie, 5 Rue J B
C1ément, F-92290 Chatenay Malabry, France,
‡Hôpital R. Debré U62, Rue A. Carrel, F-51092
Reims Cédex, France; § Laboratoire de
Physiopathologie du Développement, Groupe Interactions
Cellutaires, Univ. P. Et M. Curie 4 Place Jussieu, F-75252 Parts
Cédex 05, France
Summary: The nervous form of magnesium
imbalance represents the best documented experimental and
clinical aspects of magnesium disorders. The nervous form of
primary magnesium deficit (MD) in the adult appears as the best
descriptive model for analysis of the symptomatology,
aetiology, physiopathology, diagnosis and therapy of the most
frequent form of MD. Nervous hyperexcitability due to chronic
MD in the adult results in a non-specific clinical pattern with
associated central and peripheral neuromuscular symptoms,
analogous to the symptomatology previously described in medical
literature as latent tetany, hyperventilation syndrome,
spasmophilia, chronic fatigue syndrome, neurocirculatory
asthenia and idiopathic Barlow's disease.
On encountering this non-specific pattern, the signs of
neuromuscular hyperexcitability are of much greater importance.
Trousseau's sign is less sensitive than Chvostek's sign, but
their sensitivities, are increased by hyper- ventilation (Von
Bondsdorff's test). Examination of the precordial area will be
conducted in order to search clinical stigmata of mitral valve
prolapse (MVP) which is a frequent dyskinesia due to chronic MD
(about a quarter to one- third of cases).
The electromyogram (EMG) shows one (or several) trains of
autorhythmic activities beating for more than 2 min of one of
the three tetanic activities (uniplets, multiplets or 'complex
tonicoclonic tracings') during one of the three facilitation
procedures: tourniquet-induced ischaemia lasting 10 min,
post-ischaemia lasting 10 min after the removal of the
tourniquet and hyperventilation over 5 min. A repetitive EMG
constitutes the principal mark of nervous hyperexcitability
(NHE) due to MD. The echocardiogram (ECC) is the best tool for
detecting MVP, the 2-dirnensional ECC with pulsed Doppler being
more accurate than time-motion ECC.
The routine ionic investigations comprise five static tests:
plasma and erythrocyte magnesium, plasma calcium and daily
magnesiuria and calciuria. An evaluation of magnesium intake is
desirable. Normal concentrations of magnesium in blood do not
rule out the diagnosis of the nervous form of primary chronic
MD. The histograms of MD group reveal Gaussian type
magnesaemias with significantly lower means and the constituent
elements can be individually hypo- (one-third of cases), normo-
(about two-thirds of cases) and even, exceptionally,
hyper-magnesaemic. The diagnosis of MD requires an oral
magnesium load test. At physiological dose (5 mg of Mg/kg/day),
oral magnesium is totally devoid of the pharmacological effects
of parenteral magnesium. Corrections of symptomatology by this
oral physiological magnesium load is the best proof that it was
due to magnesium deficiency.
In particular clinical forms, more sophisticated studies may
be useful: standard and quantitative electroencephalograms
electropolygraphic studies of afternoon sleep,
electronystagmography, optokinetic test, skin conductance
reflex, psychometric inventories, standard or monitoring
electrocardiogram, treadmill test, other static and dynamic
investigations: e.g. ionized free Mg 2+, lymphocyte
Mg, brain Mg, cerebrospinal Mg, Mg balance, Mg parenteral load
test, glucose load, and even radio-isotope study, the only one
able to reveal intestinal magnesium hypersecretion.
Nervous primary chronic MD progresses by phases of
decompensation against a background of latency.
Marginal magnesium deficiency, that is to say an
insufficient magnesium intake which merely requires simple oral
physiological supplementation, is fundamental in the astrology
of primary magnesium deficit. However a constitutional
homeostatic lability of the nervous system or of magnesium
metabolism such as belonging to the B35 type of HLA group must
be involved. Part of the aetiology of this magnesium deficit is
a magnesium depletion, where the disorder which induces
magnesium deficit is related to a dysregulation of the control
mechanisms of magnesium status which requires a more or less
difficult specific correction.
MD induces diffuse NHE.
Symptomatic forms result in three direct cellular effects:
disturbances in Ca distribution, decreased second messenger
nucleotidic ratio and increased susceptibility to peroxidation.
NHE is also linked to local and systemic mediated effects:
increased activity of excitatory neuromediators with a
decreased activity of inhibitory neuromediators, increased
production of inflammatory and immunostimulant mediators:
neuropeptides, prostanoids, cytokines and of prooxidant factors
with decreased antioxidant system. These factors induce Ca i,
↑ cAMP/cGMP and ↑ polarization which cause the
symptomatic form of nervous MD.
Local compensatory factors instrumental in the latency of
NHE due to MD may also be direct and indirect: in the cell
through modifications of Ca and Mg binding proteins, increase
of Mg-like polyamines, stimulation of antioxidant system and
free radical scavengers, local and systemic mediated
compensatory factors: increase in several neuroprotective
agents, increased production of anti-inflammatory and
immunosuppressive mediators, with decreased pro-oxidant factors
and an increased anti-oxidant defence.
It seems very important to highlight the difference between
the pathophysiological mechanisms of magnesium deficiency and
of the different types of magnesium depletion: genetic and
acquired, irreversible (or partially reversible) and,
reversible. These last models of depletion may constitute
promising tests for screening treatment both in these magnesium
depletions and in the related type of disorders such as some
neurodegenerative diseases.
There is no pathognomonic sign of nervous MD. However, the
diagnosis is obvious before Chvostek's sign and/or tetanic EMG
tracings without hypocalcaemia and/or hypercalciuria or a
pattern of idiopathic PVM. Positivity of the dynamic oral
physiological magnesium load test constitutes the proof of a
magnesium deficiency. Usually it is very easy to distinguish
between primary or secondary MD, because the symptomatology of
the causative disorder is clear. However, two eventualities
raise problems: 1. MD secondary to hyper-calciuria, where
systematic dosage of daily calciuria is necessary: 2. MD
secondary to neurosis. Metabolic and psychological phenomena
may be closely interrelated with mutual aggravation. There are,
genuine 'neuromagnesiprive forms' where it is difficult to
determine the predominance of neurosis and MD factors.
Physiological oral magnesium supplementation (5 mg/kg/day)
is easy and can be carried out in the diet (using preferably
high magnesium density nutrient with the best possible
availability, particularly magnesium in water) or with
magnesium salts, with practically only one contraindication:
overt renal failure (creatinine clearance < 15 ml). Specific
and non-specific treatments of magnesium depletion are tricky
when using, for example, pharmacological doses of vitamine B6,
physiological doses of vitamine D, magnesium sparing diuretics,
partial magnesium 'analogues', antioxidants i.e. sulphur
compound, vitamin E, selenium.
Clinical forms of nervous primary MD are multiple. There is
a great variety of symptomatic forms according to the main
target of the symptomatology: migraine, chronic fatigue,
neurosis, restless leg syndrome, Raynaud's phenomenon with an
increase of CGRP Mg-dependent, neurolability in children and
attention deficit/hyperactivity disorders (ADHD). Undetected
early maternal magnesium deficiency could be the fountainhead
of the so called 'constitutional' characteristics of the
nervous form of chronic primary MD, and even of more severe
impairments: sudden infant death syndrome, some forms of
infantile convulsions or psychiatric disturbances and even in
adults cardiovascular diseases and non-insulin-dependent
diabetes mellitus. The protocol of the multicentre trials of
maternal magnesium supplementation should be followed not only
in the mother, the fetus, and the neonate, but also in the
child and even throughout life from infancy until older
age.
The secondary forms of MD can be spontaneous or iatrogenic.
Their therapy requires first treatment directed toward their
specific aetiologies. When this proves impossible or
inefficient the treatment of each type of secondary MD will
differ according to the type of MD. If deficiency predominates,
e.g. frequently in chronic alcoholism -- a mere oral
physiological magnesium supplementation will be appropriate.
When MD secondary to alcoholism has been corrected, no
alcoholic encephalopathy will be observed within a period of
five years. If depletion predominates -- e.g. in diabetes
mellitus -- the specific or non-specific treatment of magnesium
status dysregulation should be mainly used.
Symptomatic magnesium excess (ME) is practically always
iatrogenic either by magnesium therapy in spite of renal
failure or during massive parenteral magnesium therapy.
Clinical signs appear only when plasma magnesium is increased
2-3 times. Drowsiness and hyporeflexia are observed first. It
is only when magnesaemia is at least five times higher that
areflexia is observed which precedes respiratory paralysis.
The treatment first to stop latent or hidden administration
of magnesium. Afterwards the therapy should use the classical
antidote intravenous Ca, artificial respiration, osmotic
diuresis, anticholinesterasic drugs and cardiac glycosides, and
lastly extrarenal dialysis.
Latent hypermagnesaemia may be observed in various,
iatrogenic and pathogenic circumstances: inadequate excretion
in chronic renal insufficiency, dysregulation of magnesium
metabolism in various nervous, endocrine and immunological
impairments such as some depressions, phaeochromocytomes,
respiratory acidosis, systemic diseases, cancer,
pharmacological magnesium therapy. It is of basic importance to
contrast the non toxicity of the physiological oral magnesium
supplementation and the possible toxicity of pharmacological
uses of magnesium in therapeutics, which is potentially
dangerous and possibly lethal.
Key words: Nervous hyperexcitability,
magnesium, deficiency, depletion, Chvostek's sign,
electromyogram, mitral valve, prolapse, magnesium loading
tests, acetylcholine, excitatory aminoacids, taurine,
cytokines, neuropeptides, oxygen free radicals,
neurodegenerative diseases.
Introduction
The nervous forms of magnesium imbalance represent the best
documented experimental and clinical aspects of magnesium
disorders.1-8
Magnesium excess is almost exclusively a complication of
therapy: it is practically always of iatrogenic origin.
The nervous forms of magnesium deficit exhibit extreme
clinical polymorphism and present difficult diagnostic,
physiopathologic and therapeutic problems. But whatever the age
they are the most commonly seen forms of magnesium deficit in
clinical practice.1-8
Study of magnesium deficit (MD) is an important aspect of
clinical medicine today. We will take the nervous form of primary
magnesium deficit in the adult as the descriptive model for
analysis of the symptomatology, aetiology, physiopathology,
diagnosis and therapy of the most frequent and characteristic
form of MD. We will further study some clinical forms of nervous
primary and secondary magnesium deficit. Finally we will deal
with magnesium excess.
Nervous form of primary chronic MD
Nervous hyperexcitability due to chronic MD in the adult
results in a non-specific clinical pattern which associates
central, peripheral and autonomic neuromuscular symptoms,
analogous to the symptomatology previously described in medical
literature under various names, mainly latent tetany,
hyperventilation syndrome, spasmophilia, chronic fatigue
syndrome, neurocirculatory asthenia, idiopathic Barlow's disease
and Da Costa syndrome. According to the subject, the
etiopathogenic hypotheses and the main symptomatology, many other
denominations have been used: Scythian disease, Icelandic,
disease, Akureyry disease, Royal Free disease, Yuppies syndrome,
neurolability, hyperactivity disorder without (HD) or with
attention deficit (ADHD hyperkinetic syndrome; hypochondriasis,
hysteria, melancholia, neurasthenia, depression, anxiety;
cryptotetany, functional disorders, neurovegetative disorders,
dysautonomic. disorders, reactive hypoglycemia,
pseudo-myasthenia, epidemic neuromyasthenia, myalgic
encephalomyelitis, post-viral syndrome, chronic mononucleosis,
chronic candidosis, total allergy; functional cardiac disorders,
irritable heart, soldier's heart, effort syndrome, labile blood
pressure, Raynaud's syndrome; functional digestive disorders,
irritable bowel syndrome, biliary dyskinesia; allergic or
pseudo-allergic disorders, seasonal rhinitis,
pseudo-asthma.9-20
Subjective symptomatology
The symptoms of nervous primary chronic MD in the adult
include non specific central peripheral and autonomic
manifestations of neuromuscular hyperexcitability.1-8,
21-38
Central or rather psychiatric symptoms consist of anxiety,
hyperemotionality, fatigue, headaches (and sometimes migraine),
insomnia, light-headedness, dizziness, nervous fits (panic attack
particularly), lipothymiae, sensation of a 'lump in the throat',
of 'nuchalgia' and 'blocked breathing'. Personality disorders are
of neurotic type.
Neuromuscular disturbances symptoms are acroparaesthesiae,
cramps, muscle fasciculations and myalgiae occurring more
frequently than tetanoid or tetanic attack.
Autonomic functional complaints include chest pain, sine
materia dyspnoea, blocked respiration, asthma-like dyspnoea,
hepatobiliary dyskinesia, gastrointestinal spasms, precordialgia,
palpitations, extrasystoles, dysrhythmias, Raynaud's syndrome,
trends to orthostatic hypotension or conversely to borderline
hypertension. In fact, the dysautonomic disturbances involve both
the sympathetic and the parasympathetic systems: neurovegetative
disorders may be amphotonic, alpha or beta sympathicotonic,
vagotonic, with reactive hypoglycemia, pseudo allergic through
hyper- receptivity to histamine and/or acetylcholine, sometimes
with genuine allergy (Type I mainly).
When the chest pain mimics coronary heart disease, its relief
with propranolol and worsening by nitrates may help to
distinguish between a benign disorder and one with a coronary
origin.
The evolution may be studded with various acute paroxysmal
manifestations which can also sometimes be seen as initial signs
of the illness. The major crises of acute tetany or of grand mal
-- even reduced to a simple loss of consciousness -- remain,
relatively rare. It is more often a question of nervous crises:
neurotic, from the, 'attack of nerves' to the 'hysterical
crises', or autonomic: lipothymia, reactive hypoglycaemia,
pseudo-asthmatic crisis, vago-vagal syncope or, on the contrary,
paroxysmal tachycardia. Sometimes, centripetal tingling
sensations and stiffness of the extremities confer on these
nervous crises a tetanoid character. But, essentially, they all
have in common the fact that they occur in the context of fits of
anxiety, sometimes with the impression of imminent death (panic
attack), which cause hyperventilation gaseous alkalosis and self
perpetuation of the crises.
Physical examination
On encountering this non-specific pattern the signs of
neuromuscular excitability are of much greater importance. A
genuine Chvostek's sign must be systematically sought. With a
small (children's) reflex hammer the examiner percusses the soft
parts of the cheek at the centre of a line running from the ear
lobe to the labial commissure, avoiding the lightning contraction
of a 'false Chvostek's sign' by tapping the bone of the zygomatic
apophysis. It is important to consider the quality, and not the
intensity, of this clinical criterion of neuromuscular
hyperexcitability. It is only its presence or its absence which
is significant, respectively quoted 1 or 0. Trousseau's sign is
less sensitive than Chvostek's sign, but both sensitivities are
increased by hyperventilation (Von Bonsdorff's test). Examination
of the precordial area should be carefully conducted in order to
search either for a non-ejection systolic click, or for a mid- to
end-systolic or pansystolic murmur, or both, particularly in
orthostatism in complete expiration in the left lateral decubitus
position. Mitral valve prolapse (MVP) is a frequent dyskinesia
due to chronic MD (about a quarter to one-third of cases).
Tracings
Routine tracings
Two tracings are always made: a neurophysiological examination
(electromyogram (EMG)) and a cardiological examination
(echocardiogram, (ECC)). In EMG testing for latent tetany, a
Bronck's needle is inserted into the first-dorsal interosseous
muscle of the left hand. The three classical facilitation tests
are used: tourniquet-induced ischaemia lasting 10 min,
post-ischaemia lasting 10 min after removal of tourniquet and
lastly hyperventilation lasting 5 min. If the EMG shows one (or
several) train(s) of autorhythmic activities, 'beating' for more
than 2 min of one of the three tetanic activities (uniplets,
multiplets or complex-tonicoclonic tracing(s)) a positive
response is defined. As determined for the clinical criterion of
nervous hyperexcitability, this neurophysiological criterion is
only considered as a two-class variable. Either its presence or
its absence is significant, respectively quoted 1 or 0 (Fig. 1).
A repetitive EMG constitutes the principal mark of nervous
hyperexcitability due to MD.
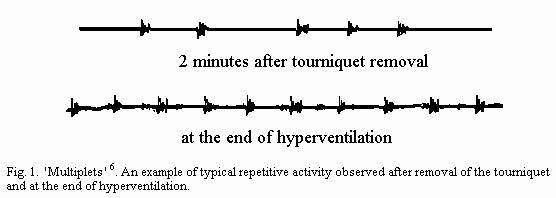
The 'excitability index' (EI) is defined as the sum of the two
criteria of tetany. It allows different classes to be
distinguished: one with simultaneous clinical and
neurophysiological criteria, EI = 2; the others with only one
criterion of their tetanic state, EI = 1, with two subgroups,
either clinical (through positivity of the Chvostek's sign
alone), or electromyographic (through positivity of EMG
alone).
The ECC is the best tool for detecting mitral valve prolapse
(MVP). With time-motion (TM) mode, three tracings are classical:
a 'cuplike' tracing of mesotelesystolic MVP (of more than 2 mm)
(Fig.2); a 'hammocking' tracing of holosystolic MVP (of more than
3mm) (Fig.3); and an isolated systolic anterior motion (SAM)
(Fig.4) observed without obstruction or any septal thickening
sign and in the absence of false systolic anterior motion.
Two-dimensional echocardiography appears to be more accurate
than TM echocardiography. It eliminates a number of artefacts,
particularly in the section of parasternal longitudinal cut
(Fig.5) and the apical cut of the four heart chamber (Fig.6). The
criterion for mitral prolapse is the billowing of one or both
leaflets below the level of the mitral ring. It is very important
to assess the leaflet thickness as well as its whole morphology
and to appreciate the ventricular kinetic by calculating:
| |
end diastolic diameter - end systolic diameter |
| ΔD = |
|
| |
end diastolic diameter |
Pulsed Doppler echocardiography allows the detection of
associated mitral regurgitation.
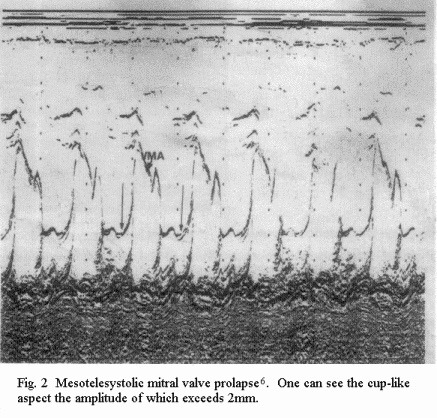
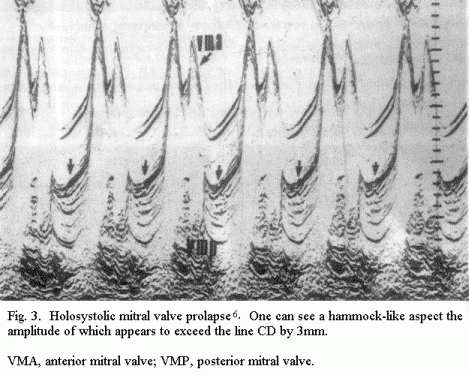
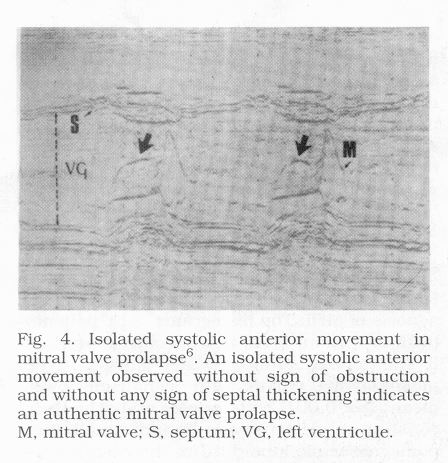
Particular clinical forms
In particular clinical forms, more sophisticated
neurophysiological or cardiological explorations may be useful.
Additional neurophysiological examinations can be used in the
evaluation of the psychic and neuromuscular condition, i.e.,
standard and quantitative electroencephalogram,
elelectropolygraphic study of afternoon sleep,
electromystagmography, optokinetic test, skin conductance reflex,
head scan and psychometric investigations such as Minnesota
Multiphasic Personality Inventory (MMPI) or evaluation of A/B
behaviour pattern by Jenkin's questionnaire.
Additional cardiological examinations may allow a better
appreciation of the cardiac condition, i.e. standard and/or
effort electrocardiogram, ambulatory electrocardiogram (Holter),
phonocardiogram, or, very exceptionally, angiocardiogram.
Ionic evaluation
Routine ionic assessment
Five ionic static investigations should always be made: plasma
Mg (pMg), erythrocyte Mg (eMg), calcaemia, daily magnesuria and
daily calciuria which can be determined by testing for urinary
infection. An evaluation of magnesium intake through dietary
inventory is desirable. These must first demonstrate
normocalcaemia and the absence of hypercalciura sufficient to
induce a secondary magnesium deficit. Next, the evaluation of pMg
and eMg with reliable methods, such as atomic absorption
spectrophotometry, allows the diagnosis of primary magnesium
deficit through hypomagnesaemia in one-third of the cases of
latent tetany due to MDI, with or without MVP (Table 1). Normal
levels do not rule out the diagnosis of MDI. The histograms of LT
patients (with or without MVP) and of controls overlap. If the
tetanic group reveals gaussian-type magnesaemia curves with
significant lower means (p < 0.001) both for pMg and
eMg, their constitutive elements can be individually
hypomagnesaemic (one-third of the cases), normomagnesaemic
(almost two-thirds of the cases) and even, although seldom,
hypermagnesaemic (Fig.7). Nevertheless one must emphasize the
remarkable constancy of magnesaemia which lends importance even
to small variations of magnesaemia. In numerous cases
(particularly those with normal parameters) the diagnosis
requires a magnesium oral loading test. The dose of magnesium to
be administered is 5mg/kg/day of a well absorbed salt for a least
1 month. At this physiological dose level, oral magnesium is
totally devoid of the pharmacodynamic effects of parenteral
magnesium. Correction of symptoms by this oral magnesium load
constitutes the best proof that they were due to magnesium
deficiency, as checked after one month of treatment. However, a
negative response does not permit rejection of a diagnosis of
magnesium deficit. A mere increase of the magnesium intake is
sometimes inadequate to ensure uptake or maintenance of cellular
magnesium, in forms of deficit corresponding to magnesium
depletion due to a dysregulation of the factors which control its
metabolism. Therefore, if the response remains negative after 1
month's physiological magnesium oral loading test, it should be
followed by use of the best specific control of the Magnesium
metabolism dysregulating factors. Otherwise the investigation may
be continued with the addition of a non-specific magnesium fixing
agent to the magnesium salt: for example pharmacological doses of
vitamin B6, physiological doses of vitamin D metabolites or of
Mg-sparing diuretics able to reduce a possible hypermagnesuria
(Table 2).
Thus pMg, eMg, calcaemia and calciuria are the first minimum
ionic measurements required. They are often completed by checking
daily magnesuria in order to control the effectiveness of the
magnesium oral loading test after the first month of
treatment.
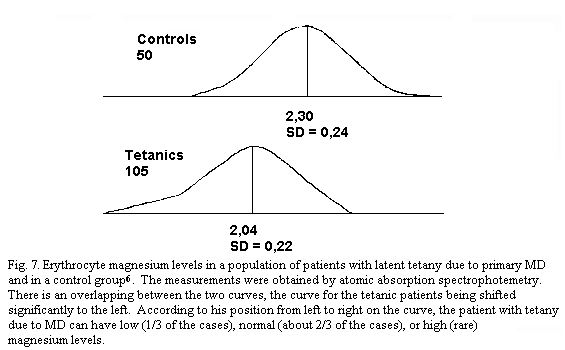
Table 1. Plasma and erythrocyte magnesium
concentration in control group and in a hypomagnesemic
population of patients with latent tetany due to primary
magnesium deficit6
|
|
| |
| |
Control |
|
Hypomagnesemic |
|
| |
Plasma Mg |
|
Erythrocyte
Mg |
|
Plasma Mg |
Erythrocyte Mg |
| |
x |
SD |
|
x |
SD |
|
x-2SD |
x-2SD |
| mmol L |
0.88 |
0.05 |
|
2.30 |
0.24 |
|
0.78 |
1.82 |
| mg L |
21.40 |
1.14 |
|
55.94 |
5.96 |
|
19.12 |
44.02 |
|
| Table 2. Comparison
between oral parenteral magnesium load test |
|
| |
|
|
|
|
| Method of administration |
|
Oral |
|
Parenteral |
| Dose |
|
Physiological |
|
Pharmacological |
| Administration |
|
Ambulatory |
|
In hospital |
| Criteria of evaluation |
|
|
|
|
|
Clinical |
|
+ + + |
|
0 |
|
Biological |
|
+ + + |
|
+ + + |
| Duration |
|
Several weeks |
|
4 h |
|
|
Physiological magnesium oral load test constitutes the best
proof that the clinical pattern is due to magnesium
deficiency and the first step of its specific treatment.
Parenteral magnesium load test serves as a rapid diagnosis
of magnesium deficit, but without diagnostic
differentiation between magnesium deficiency and magnesium
depletion. As the clinical effect of parenteral
pharmacological doses might be purely pharmacodynamic the
results can be only assessed on objective criteria. |
Particular clinical forms
More sophisticated studies may be carried out to determine
magnesium or other metabolites. Some studies are static:
magnesium balance, ionized and diffusible fractions of plasma
magnesium, levels of magnesium in cerebrospinal fluid,
lymphocytes, bone, muscle, even platelets and exceptionally
myocardium and brain. Other investigations are dynamic. The most
useful is the parenteral magnesium load test, for example,
Thoren's technique, the efficiency of which is judged by the
modifications of the magnesuria 4 hours after the infusion of
0.25 mM (about 6mg/kg/day). Thus a retention which exceeds 20 per
cent of the magnesium supplied, corresponding to a magnesuria
lower than 80 per cent of the parenteral load, supports the
diagnosis of magnesium deficit without diagnostic differentiation
between deficiency, and depletion (positive Thoren's test). This
parenteral load test serves as a rapid diagnosis of deficit, but
it can only be carried out in hospital. As the clinical effects
of parenteral pharmacological doses might be purely
pharmacodynamic, the results can only be assessed on the basis of
objective criteria. Finally a negative Thoren's test may be
observed in cases of depletion ('false negative response' for
this form of magnesium deficit), as well as in cases of a
balanced metabolism ('true negative response' (Table 2).
Evaluation of modifications of the magnesaemia during a
standardized effort (a treadmill test and/or a mental task),
after glucose or calcium, or phosphorus loads and, exceptionally,
radioisotopic exploration may complete investigations into
magnesium balance; it is the only one able to reveal magnesium
intestinal hyperexcretion. Other metabolites may be
simultaneously explored in plasma, erythrocyte, blood, urine,
such as potassium, zinc, iron, phosphorus, phosphatases, ATP,
creatine, creatine kinase, glucose, aminoacids, essential fatty
acids.
Complete record
In rare cases, it may be interesting to evaluate both the
neuroendocrine factors of magnesium homeostasis and the main
disrupting elements. These include on the one hand measurements
of adrenaline, noradrenaline, PTH, CT, insulin, taurine, cyclic
AMP and GMP and/or, on the other hand, measurements of
angiotensin, renin, aldosterone, vitamin D metabolites, free T3
and T4. Sometimes, it may be useful to investigate immunological
data such as neuropeptides, cytokines, peroxidant and antioxidant
factors, immunoglobulin levels, histamine, acetylcholine,
noradrenaline, isoprenaline receptivities, and haemorrheological
data such as platelet and erythrocyte explorations, familial data
(history, HLA typing etc.).
Evolution and prognosis
Usually, the prognosis of the neuromuscular form of MDI is
favourable, The evolution progresses by phases of decompensation
against a background of latency. It appears of major importance
to distinguish between the larger group of patients (95 per cent
of the cases) with a benign prognosis and even with the
possibility of complete recovery, and the smaller subgroup of
patients (5 per cent of the cases) running the risk of
complications. In this latter population, MD appears as a nervous
and cardiovascular risk factor more prone to arrhythmias, sudden
death, endocarditis, cerebral or visual or inner ear ischaemic
events. Among the main factors of a favourable prognosis are
latency or paucity of clinical and paraclinical symptomatology:
i.e. low excitability index (IE = 1), absence of auscultatory
signs, non-redundant and thin leaflets, absence of mitral
regurgitation, normal weight, no oestrogen intake and lastly the
deficient type of the magnesium deficit with its simple
treatment.
Conversely, it is important to determine some pejorative
prognosis factors, i.e. a rich symptomatology particularly with
ventricular arrhythmias, a high excitability index (IE = 2), not
so much a click as mitral regurgitation stigmata, redundant thick
mitral leaflets especially in older men, prolongation of QTc
interval, underweight, thrombogenic disturbances mainly through
alterations of the platelet function, immunological disorders,
constitutional factors such as the carrying of the HLA B35
antigen, exceptionally a familial history of sudden death and
lastly the depletion type of the magnesium deficit with its often
difficult and sometimes prolonged treatment.5
Aetiology of the nervous form of primary chronic
magnesium deficit : magnesium deficiency and magnesium
depletion
Marginal magnesium intake is fundamental in the causation of
primary chronic magnesium deficit. Magnesium deficiency merely
requires simple oral magnesium supplementation1-8, 26, 30,
39 , but cannot entirely explain the etiology of the
nervous form of primary chronic magnesium deficit.
Magnesium deficiency alone cannot account for the following
four observations: (1). the prevalence among the population
(15-20 per cent); (2) the variable time of occurrence of
therapeutic improvement; (3) the frequency of total or partial
failure of treatment; (4) the constitutional character of the
disease, associated with a strong heritability of erythrocyte
magnesium and a strong correlation between the HLA-B35 tissue
antigens either with latent tetany or mitral valve prolapse or
erythrocyte magnesium40-42 . A constitutional lability
of the nervous system or of magnesium metabolism must be
involved: it' constitutes the part of magnesium depletion in this
magnesium deficit requiring a more or less specific
correction.1-8, 40-42
Physiopathology
The mechanisms which control nervous hyperexcitability in
magnesium deficit have been better analyzed in magnesium
deficiency than in magnesium depletions8, 43 . The
blood-brain barriers reduce the importance of peripheral systemic
controls. In magnesium deficit diffuse nervous hyperexcitability
mainly derives from local mechanisms.
The nervous form of primary chronic magnesium deficit may be,
in both clinical circumstances and experimental deficiencies,
either patent with a nervous symptomatology or latent without any
clinical manifestations. A general scheme of the factors
controlling nervous hyperexcitability due to magnesium deficit
will explain both symptomatic and latent clinical forms.
Extrapolating from data in vitro, in situ or from
other pharmacological manipulations to the physiopathology of
in vivo magnesium deficit remains a
methodological error. The great stability of brain magnesium
during magnesium deficiency particularly disagrees with the very
notion of extrapolating from in vitro or in
situ extracellular or intracellular magnesium modifications.
The complexity of biology must not be disregarded just because
the present trend focuses on one aspect of the knowledge at the
expense of many others. For example the great attention given to
studies on magnesium and NMDA receptors should not minimize the
interest of many other mechanisms.44
General scheme of nervous hyperexcitability due to
magnesium deficiency8, 43 .
Symptomatic forms result in three direct cellular effects:
disturbances in Ca distribution, decreased second messenger
nucleotidic ratio and increased susceptibility to peroxidation.
NHE is also linked to local and systemic mediated effects:
increased activity of excitatory neuromediators; neuroamines
(acetylcholine, catecholamines) and aminoacids (NMDA, AMPA,
KAINATE), with a decreased activity of inhibitory neuromediators:
serotonin 45 , aminoacids (GABA and taurine (TA)
mainly), adenosine, melatonin and opioids accessorily, increased
production of inflammatory and immunostimulant mediators;
neuropeptides, (substance P, CGRP, VIP), prostanoids (LTB4, PXB2,
PGE2), cytokines (NO, IL1, IL6, TNF# ) and of prooxidant factors
(aldehydes such as MDA, TBARS, Fe) with decreased antioxidant
system ( ↑ GPX, ↑ MT, ↑ Vitamin E, ↑ Se,
↑ TA). These factors induce → Ca i, ↑ cAMP / cGMP
and ↑ polarization which cause the symptomatic form of
nervous magnesium deficit.
Local compensatory factors instrumental in the latency of NHE
due to magnesium deficit may also be direct and indirect: in the
cell through modifications of Ca and Mg binding proteins,
increase in Mg-like polyamines, stimulation of antioxidant system
and free radical scavengers, local and systemic mediated
compensatory factors: increase in several neuroprotective agents
( TA mainly, GTA and → MT), increased production of
anti-inflammatory and immunosuppressive mediators (such as →
IL4, IL5, IL10, → IFN) with decreased pro-oxidant factors (
↑ MDA, ↑ TBARS, ↑ Fe) and increased antioxidant
defence ( SoD, → MT, vitamin E, Se, → TA).
General scheme of nervous hyperexcitability due to
magnesium depletion
Little is known yet of the mechanisms of magnesium depletion.
Because of its links with causal dysregulations from various
origins there exists a great variety of clinical and experimental
magnesium depletions. Diverse experimental models of unequally
severe magnesium depletions are used: genetic models in
rats46 and mice47-49 and acquired
models.
Among the latter some are secondary to an irreversible (or
partially reversible) cause such as traumatic brain
injury50-55 , blast injury56 or neurotoxic
metal load associated with Mg (and Ca)
deficiencies57-59 . Three of acquired experimental
magnesium depletions are reversible. They associate various types
of stress, capable of inducing excitotoxicity, with a low
magnesium intake. Physiological magnesium supplementation and
pharmacological doses of Na are acetyltaurinate are ineffective,
but Mg acetyltaurinate has preventive and curative effects, in
both the short and long term. These magnesium depletion models
may be useful for screening various drugs for treatment of this
type of magnesium depletion60-63 .
Further research should stress the differences between the
mechanisms of magnesium deficiency and those of various types of
irreversible and reversible magnesium depletions. It would be
interesting to study neuromediators and neuromodulators, their
precursors and their metabolites on the greatest possible number
of structures of the central nervous system and to consider their
correlations with ions, neuropeptides, cyclic nucleotides,
cytokines, eicosanoids, enzymes, vitamins, metabolites. For
example, as a rule (except in one study, concerning 2-month-old
Wistar rats57 ) no changes have been found in brain
magnesium concentrations during the course of magnesium
deficiency in adult rats8, 43, 64, 65 ; in the
experimental and clinical type of magnesium depletion related to
pollutant metal load and low Mg and Ca intake, magnesium
concentrations were decreased in various structures of the
nervous central system57-59 .
Diagnosis
Positive diagnosis
There is no pathognomonic sign of nervous magnesium deficit.
However, diagnosis is obvious before Chvostek's sign and/or
tetanic EMG tracings without hypocalcaemia and/or hypercalciuria
or a pattern of idiopathic PVM. Positivity of the dynamic oral,
physiological magnesium load test constitutes the proof of a
magnesium deficiency and the first stage of its therapy.
Differential diagnosis
Usually it is very easy to distinguish between primary or
secondary magnesium, deficit, be- cause the symptomatology of the
causative disorder is clear. However, two eventualities raise
problems: (1) Magnesium deficit secondary to hypercalciuria,
where systematic dosage of daily calciuria is necessary; (2)
magnesium deficit secondary to neurosis. Metabolic and
psychological phenomena may be closely interrelated with mutual
aggravation. There are true 'neuromagnesiprivic forms' where it
is difficult to determine the predominance between neurosis and.
magnesium deficit factors.
Therapy 1-8, 31
Physiological oral magnesium supplementation (5mg/kg/day) is
easy and can be carried out in the diet (using preferably high
magnesium density nutrient with the best possible availability,
particularly magnesium in water) or with magnesium salts, with
only one contraindication: overt renal failure (creatinine
clearance < 15 ml). Specific and non-specific treatments of
magnesium depletion are tricky when using, for example,
pharmacological doses of vitamin B6, physiological doses of
vitamine D, magnesium sparing diuretics, partial magnesium
'analogues', antioxidants i.e. sulphur compound, vitamine E,
selenium.
Clinical forms
The clinical forms of nervous primary and secondary chronic
magnesium deficit vary greatly according to the main target of
the symptomatology, age and gender, and to the association with
non nervous symptomatologies of magnesium deficit. As a result
there is hardly a discipline where one or another form is not to
be found.
Clinical forms of nervous primary chronic magnesium
deficit
Symptomatic forms
Among the actual nervous clinical forms, the forms with
insomnia, headache and fatigue will be stressed.
The insomniac or rather dyssomniac form1-8 can be
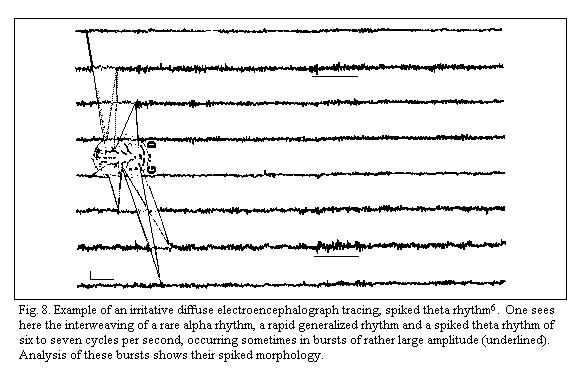
analyzed with polygraphic study. Standard and. quantitative EEG
exhibit 'diffuse irritative tracings' without focal lesions or
paroxysmal, discharges. The recordings contain spikes, a pointed
appearance of alpha and/or theta waves which is facilited more
often by hyperventilation than by intermittent photic simulation,
(Fig.8)1-8 . Quantitative analysis of the EEG effects
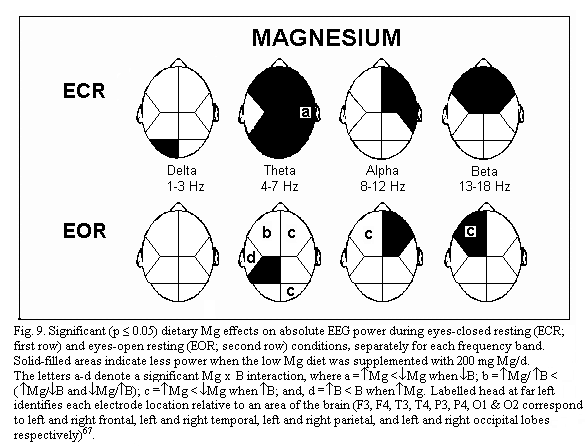
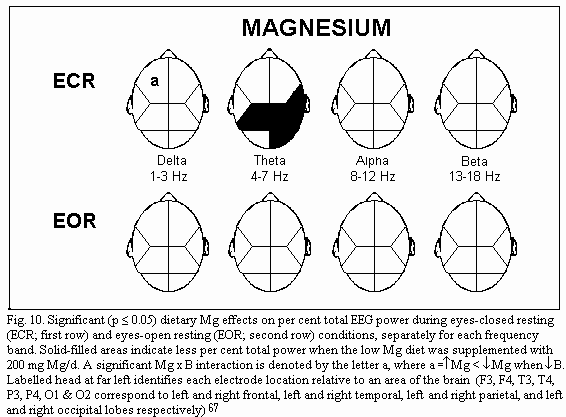
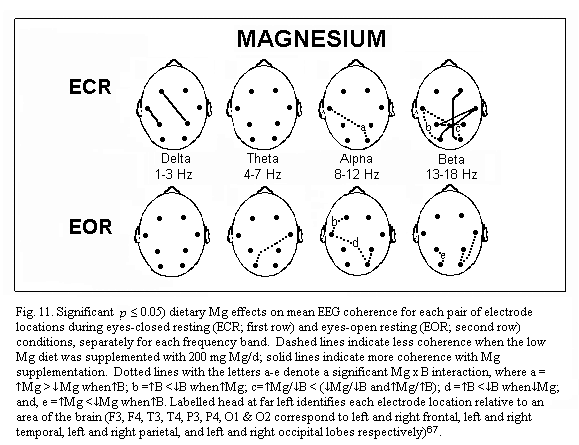
of marginal magnesium deficiency66-67 shows three main
points. During eye closed resting condition, absolute power is
increased in several brain regions (frontal regions, right
temporal and parietal regions) (Fig.9); frequency specific power
is increased for each frequency band (delta, theta, alpha and
beta) differently in various brain regions: for example theta
power is increased in all but the left temporal regions (Fig.10);
mean EEG coherence is decreased (Fig.11). Simple oral
physiological magnesium supplementation may reverse these effects
leading to less theta activity and more EEG
coherence67 . The polygraphic study of afternoon sleep
may complete the data of standard EEG: brevity of the time to
fall asleep, superficial character of the sleep, frequency of
awakening, hypnoagnosia. This dyssomnia must be responsible for
morning asthenia. Electrocorticography in the magnesium-deficient
rat allows us to make observations similar to those found in
humans: sleep quality shows particularly similar alterations of
the hypnograms2-8,68,69 .
Nervous hyperexcitability due to magnesium deficit may
stimulate various types of cephalalgia tension-type headaches and
particularly migraine1-8,21-38,70-71 . Clinical,
studies in migraineurs have shown heterogeneous and inconstant
decreases in extra or intra-cellular, total or ionized magnesium
concentrations in serum, saliva, erythrocyte, mononuclear cells,
even in brain. Positive therapeutic response to oral magnesium
physiological load is unreliable2-8,20-38,70-84 .
These data agree with some possible dysregulation of magnesium
status in migraine (magnesium depletion which requires a more or
less specific correction of the dysregulation). But when chronic
magnesium deficiency coexists with migraine, it only constitutes
a decompensatory factor whose control with simple oral
physiological, magnesium supplementation should help in migraine
therapy as an adjuvant treatment. Magnesium deficiency does not
constitute the cause of migraine per se.
The clinical form of nervous primary chronic magnesium deficit
with predominance of asthenia defines an aetiopathogenic form of
chronic fatigue syndrome (CFS). Instead of the imprecise notion
of CFS devoid of heuristic content the symptom of fatigue might
be investigated through an aetiological approach as a simple
clinical form of chronic primary magnesium deficit. The
subjective symptomatology of chronic primary magnesium deficiency
may, be similar. The diagnosis must rely on a magnesium oral
loading test. At the physiological dose, (5mg/kg/day) oral
magnesium is totally devoid of the pharmacodynamic effects of
parenteral magnesium which may be observed irrespective of the
magnesium status. Criteria for evaluation of the results of this
oral, magnesium load checked monthly must concern not only
magnesium parameters such as erythrocyte magnesium concentrations
but also the whole clinical and biological pattern e.g. on the
Nottingham health profile 38,85-87 .
Psychiatric forms of magnesium deficiency have been well
identified1-8, 21-38, 88-89. Personality disorders are of the
neurotic type. For example the Minnesota Multiphasic Personality
Inventory (MMPI) finds a direct correlation between the 'neurotic
triad' (hypochondria, depression, and hysteria) and the EMG marks
of NHE due to magnesium deficiency. With all the psychometric
evaluations, and with the DSM III R interview, particularly, the
clinical pattern induced through magnesium deficiency was always
neurotic (for example: generalized anxiety, panic attack
disorders, and depression) but never psychotic. Magnesium
deficiency never induces dementia. Although a neurosis pattern
due to deficiency is frequently observed and simply cured through
oral physiological supplementation, neuroses are preeminently
conditioning factors for stress. Neuroses may therefore very
frequently produce secondary magnesium depletion. They require
their own specific antineurotic treatment and not mere oral
magnesium physiological supplementation, but genuine forms of
neurosis due to primary neural magnesium deficiency and magnesium
depletion secondary to a neurosis may both exist. These two
conditions may be concomitant and reinforce each other. In these
stressful patients it may be difficult to establish the primary
of one or of the other. In practice, physiological oral magnesium
supplements may be added to psychiatric treatments, at least at
the start. Although the psychiatric forms of magnesium deficiency
may fit into a neurotic pattern but never result in dementia,
some types of magnesium depletion can be instrumental in the
physiopathology of several types of dementia 8 .
Garden soil and drinking water in some Western Pacific areas with
high incidence of amyotrophic lateral sclerosis and
parkinsonism-dementia (ALS-PD) contain high concentrations of
polluting metals such as Al, Fe and Mn and low concentrations of
common metals such as Mg and Ca. Decreased exposure to
traditional sources of foodstuffs and drinking water resulted in
a dramatic decline in ALS-PD. These data as well as the links
between aluminum load, magnesium status and dialysis
encephalopathy -- hypothetically, Alzheimer's disease --
highlight the interest of corresponding experimental studies.
With a high Al diet alone, Al content in the nervous system in
rats showed no difference with a control group although serum Al
was high. No degenerative process was observed. However, with an
insufficient intake of Mg (with or without an insufficient intake
of Ca) the same Al load induced an increase in Al and Ca
concentrations in the nervous system and neurodegeneration with
precipitation of insoluble hydroxyapatites 57-59 .
The dysregulations of magnesium status can not currently be
controlled either in Alzheimer's disease, dialysis encephalopathy
and ALS-PD or in corresponding irreversible magnesium depletions
models 8,57-59,90 . Nevertheless the description of
three possibly reversible models) of magnesium depletion, induced
by the association of excitotoxicity and low magnesium intake
appears very important: magnesium acetyltaurinate has preventive
and curative effects in both short and long
terms8,60-63 . These three models may be useful not
only for screening various treatments of this type of magnesium
depletion but also of possibly linked psychiatric and geriatric
disturbances.
Neuromuscular clinical forms of chronic primary magnesium
deficit with predominance of myalgias can appear as non articular
forms of rheumatism such as fibromyalgia whose nosological
pattern would suggest magnesium depletion with disturbances in
magnesium distribution rather, than magnesium deficiency. Simple
oral physiological magnesium supplementation is usually
inefficient91-94 . Chronic magnesium deficit might
also cause restless legs syndrome95 .
Dysautonomic clinical forms of nervous chronic primary
magnesium deficit 2-8,21-38 may be cardiovascular,
digestive or bronchopulmonary functional disorders, for example
Raynaud's phenomenon (with an increase of circulating calcitonin
gene-related peptide (CGRP) Mg-dependent)96 and
asthma-like dyspnoea (which may coexist with asthma and
constitute a decompensatory factor whose control with simple oral
physiological supplementation may help in asthma
therapy)97-98.
The dysautonomic disturbances may be amphotonic, # or #
sympathicotonic,33-99 vagotonic, with reactive
hypoglycemia, pseudoallergic through hyperreceptivity to
histamine and/or acetylcholine, and sometimes with genuine
allergy (Type I mainly)100-103 .
Clinical forms according to age and gender: risk
populations for magnesium deficit
Magnesium needs are increased during growth. Children with
attention deficit and/or hyperactivity disorder (ADHD), diagnosed
using the DSM IV criteria of this disorder, may represent a
clinical pattern of the nervous form of primary chronic magnesium
deficit104 . Nevertheless standard stimulant ADHD
therapy i.e. through dextroamphetamine significantly increases
plasma Mg, without changes in red blood cell and mononuclear
blood cell magnesium105 . Oral physiological magnesium
supplementation is an efficient treatment of the various nervous
forms of primary chronic magnesium deficiency in children: ADHD,
hyperkinetic children, latent tetany, functional neurovegetative
disorders2-8,104-107 , sometimes with speech delay or
stuttering.108
Undetected early maternal magnesium deficiency could be the
fountainhead of the so called 'constitutional' characteristics of
the forms of chronic primary magnesium deficit, and even of more
severe impairments: sudden infant death syndrome (and possibly
respiratory distress syndrome, bronchopulmonary dysplasia,
necrotizing enterocolitis, retinopathy, in premature neonate
particularly), some forms of infantile convulsions or psychiatric
disturbances; and even in adults cardiovascular diseases and
non-insulin-dependent diabetes mellitus109-118 . The
protocol of the multicentre trials of maternal magnesium
physiological supplementation should be followed not only in the
mother, the fetus and the neonate, but also in the child and even
throughout life from infancy until older age.
Geriatric89,119-124 and sport66, 125-139
populations constitute two typical magnesium deficit risk
populations where magnesium deficiency is most often associated
with magnesium depletion, but primary deficit in sports medicine
seems to be more frequently related to magnesium depletion than
to magnesium deficiency while in ageing the two mechanisms are in
most cases simply associated44,121,126 .
Clinical forms of nervous secondary chronic magnesium
deficit
The secondary forms of magnesium deficit can be spontaneous or
iatrogenic. Their therapy first requires treatment directed
toward their specific aetiologies. When this proves impossible or
inefficient the treatment of each type of secondary magnesium
deficit will differ according to the type of magnesium deficit.
If deficiency (that is to say an insufficient magnesium intake)
predominates, as frequently found in chronic alcoholism, mere
oral physiological magnesium supplementation will be
appropriates6, 31, 140-142 . When magnesium deficit
secondary to alcoholism has been corrected, no alcoholic
encephalopathy will be observed within a period of 5 years6,
8, 31, 43, 44, 143-144 . If depletion predominates, as in
diabetes mellitus, where the part of magnesium deficiency usually
appears less important than the dysregulation of magnesium
status6,145 the specific or non-specific treatment of
magnesium status dysregulation should be mainly used while oral
physiological magnesium supplementation is most often
ineffective6, 31, 145-148 .
Magnesium excess1, 3, 6, 31, 149-155
Symptomatic magnesium excess is practically always iatrogenic
either by magnesium therapy in spite of renal failure or during
massive parenteral therapy. Clinical signs appear only when
plasma magnesium is increased 2-3 times. Drowsiness and
hyporeflexia are observed first. It is only when magnesaemia is
at least five time higher that areflexia which precedes
respiratory paralysis is observed.
The treatment is first to stop patent or hidden administration
of magnesium. Afterwards the therapy should use the classical
antidote intravenous Ca, artificial respiration, osmotic
diuresis, anticholinesterasic drugs and cardiac glycosides, and
lastly extrarenal dialysis.
Latent hypermagnesaemia has been observed in various
iatrogenic or pathologic circumstances such as inadequate
excretion the case of latent chronic renal insufficiency and
dysregulation of mechanisms that control or disturb magnesium
metabolism in nervous, endocrine, metabolic and immunological
impairments. Lithium therapy appears to be effective in
manic-depressive psychosis only in the presence of magnesium. The
hypermagnesaemia observed in phaeochromocytoma testifies to the
role of adrenaline secretion in magnesium metabolism.
Hypermagnesaemia during respiratory acidosis is used in emergency
therapy of hypomagnesaemia. Levels of erythrocyte magnesium rise
during the progressive development of systemic diseases and solid
tumors. Links between symptoms such as hypoexcitability,
asthenia, bone and coagulation disorders have sometimes been
conjectured. High oral pharmacological doses of magnesium may be
instrumental in therapeutic chronic magnesium overload but with
latent toxicity which, unrecognized, is capable of reducing
lifespan31,44 . It is of basic importance to contrast
the non toxicity of the usual physiological oral magnesium
supplementation and the possible toxicity of pharmacological
magnesium therapy which is potentially dangerous and even
lethal3l-44.
Conclusion
Identification of the nervous form of magnesium deficit goes
further than mere rhetorical interest. In the presence of a non
specific pattern of hyperventilation or chronic fatigue syndrome,
of neurosis, of idiopathic tetany, of Barlow's disease or of
neurovegetative dystonia, one must have in mind primary magnesium
deficit which should be controlled by specific and atoxic
therapy. Primary or secondary magnesium deficit calls for a
differential diagnosis distinguishing between magnesium
deficiency due to an insufficient magnesium intake requiring
simple oral physiological magnesium supplementation and magnesium
depletion related to a dysregulation of the control mechanisms of
magnesium status. The various types of magnesium depletion
require more or less specific correction of their causal
dysregulation. Further research should provide a better insight
of the pathophysiological mechanisms which differentiate
magnesium deficiency from the various types of magnesium
depletion and suggest efficient treatment in experimental and
clinical magnesium depletions and in related types of pathologies
such as neurodegenerative diseases.
References
1.Durlach, J. (1976): Neurological manifestations of magnesium
imbalance. In: Handbook of clinical neurology, eds, P.J. Vinken
& G.W. Bruyn 28/2,pp.545-579. Amsterdam, New York: North
Holland.
2. Durlach, J. (1981): Deficit magnésique,
tétanie et dystonie neurovégétative.
Magnes. Bull. 3, 121-136.
3. Durlach, J. (1985): Neurological disturbances due to
magnesium imbalance. In: Metal ions in neurology and
psychiatry, eds S. Gabay, J. Harris, & B.T. Ho pp
121-128. New York: Alan R. Liss.
4. Durlach, J. & Durlach, V. (1986): Idiopathic mitral
valve prolapse and magnesium. State of the art. Magnes.
Bull. 8, 156-169.
5. Durlach, J. (1987-1988): Les rapports entre le
magnésium et le prolapsus de la valve mitrale. Rev.
Med. Fonctionnelle 20, 121-172.
6. Durlach, J. (1988): Magnesium in clinical
practice, p. 360. London, Paris: John Libbey.
7. Durlach, J. (1991): Magnesium: Clinical forms of primary
magnesium deficiency. In: Modern life styles, lower energy
and micronutrient status, ed. K. Pietrzik, pp. 155-167.
London: Springer Verlag.
8. Durlach, J. & Bac, P. (1997): Mechanisms of action on
the nervous system in magnesium deficiency and dementia. In:
Mineral and metal neurotoxicology, eds M. Yasui, M.J. Strong, K.
Ota & M.A. Verity, pp. 201-203. Boca-Raton: CRC Press.
9. Da Costa, J.M. (1871): Irritable heart: A clinical study of
a form of functional cardiac disorder and its consequences.
Am. J. Med. Sci. 61, 17-52.
10. Mackenzie, J. (1916): The soldier's heart. Br. Med.
J. 1, 117.
11. Friedman, M. (1945): Studies concerning the aetiology and
pathogenesis of neurocirculatory asthenia III. The cardiovascular
manifestations of neurocirculatory asthenia. Am. Heart
J. 30, 378-391.
12. Hardonk, J.H. & Beumer, H.M. (1976): In: Handbook
of clinical neurology, eds P.J. Vinken & G.W. Bruyn,
28/1 309-360. Amsterdam, New York: North Holland.
13. Ramsay, A.M. (1957): Encephalomyelitis simulating
poliomyelitis and hysteria. Lancet ii 1196-1200.
14. Fegan, K.G., Behan, P.O. & Bell, E.J. (1983): Myalgic
encephalomyelitis: report of an epidemic. J. R.
Coll. Gen. Pract. 33, 335-337.
15. David, A.S., Wesseli, S. & Pelosi, A.J. (1988):
Post-viral fatigue syndrome: time for a new approach.
Br. Med. J. 296, 696-699.
16. King, J.C. (1988): Hyperventilation: a therapist's point
of view: discussion paper. J. R. Soc. Med.
81, 532-536.
17. Lloyd, A.R., Wakefield, D., Boughton, C. & Dwyer, J.
(1988): What is myalgic encephalomyelitis? Lancet i,
1286-1287.
18. Kendell, R.E. (1991): Chronic fatigue, viruses and
depression. Lancet 337 160-162.
19. Shafran, S.D. (1991): The chronic fatigue syndrome.
Am. J. Med. 90, 730-733.
20. Rouillon, F., Delhommeau, L. & et Vinceneux, P.
(1996):Le syndrome de fatigue chronique. Pr. Med.
25, 2031-2036.
21. Fehlinger, R. & Seidel, K. (1985): The
hyperventilation syndrome: a neurosis or a manifestation of
magnesium imbalance? Magnesium 4,
9-136.
22. Fehlinger, R., Mielke, U., Fauk, D. & Seidel, K.
(1986): Rheographic indications for reduced cerebral
vasoconstriction after oral magnesium-medication in tetanic
patients. A double blind placebo controlled trial.
Magnesium 5, 60-65.
23. Agnoli, A., Nappi, G., Sandrini, G. & de Romanis, F.
(1989): Role of magnesium administration in neuronal
hyperexcitability syndrome. In: Magnesium in health &
disease, eds Y Itokawa & J Durlach, pp. 299-305, London,
John Libbey.
24. Fehlinger, R. (1990): Therapy with Mg salts in
neurological diseases. A critical appraisal. Magnes.
Bull. 12, 35-42.
25. Fehlinger, R. (1991): Therapy with Mg salts in the tetanic
syndrome and associated diseases: a critical appraisal. In:
Magnesium -- a relevant ion, eds B. Lasserre & J.
Durlach pp. 391-404 London, John Libbey.
26. Lasserre, B., Theubet, M.P., Spoerri, M. & Luccarini,
Y. (1991): Marginal magnesium deficiency: Cross-sectional and
follow up study in an outpatient setting. In: Magnesium -- a
relevant ion, eds B. Lasserre & J. Durlach, pp. 59-71,
London, John Libbey.
27. Nappi, G., Sandrini, G., Ruberto, G. & Antonacci, F.
(1991): Magnesium: New perspectives in clinical
neuropsychobiology. In: Magnesium -- a relevant ion, eds
B. Lasserre & J. Durlach, pp. 97-100, London, John
Libbey.
28. Coghlan, H.C. & Natello, G. (1991-1992): Erythrocyte
magnesium in symptomatic patients with primary mitral valve
prolapse: relationship to symptoms, mitral leaflet thickness,
joint hypermotility and autonomic regulation. Magnes. Trace
Elem. 10, 205-214.
29. Galland, L. (1991-1992): Magnesium, stress and
neuropsychiatric disorders. Magnes. Trace Elem.
10, 287-301.
30. Martignoni, E., Blandini, F., Costa, A., Sandrini, G.,
Verri, A.P. & Nappi, G. (1994): Modulation of noradrenergic
activity by magnesium salts in panic disorder and neuronal
hyperexcitability syndrome. Med. Sci. Res.
22, 429-430.
31. Durlach, J., Durlach, V., Bac, P., Bara, M. &
Guiet-Bara, A. (1994): Magnesium and therapeutics. Magnes.
Res. 71, 313-328.
32. Durlach, J. (1994): Primary mitral valve prolapse: A
clinical form of primary magnesium deficit. Magnes. Res.
7, 339-340.
33. Nappi, G., Sandrini, G. & Costa, A. (1995): Magnesium
and the nervous system: physiological and clinical aspects. In:
Magnesium and physical activity. ed. L. Vecchiet, pp. 117-128,
Pescara: Parthenon.
34. Lasserre, B., Spoerri, M., Theubet, M.P. & Moullet, V.
(1995): Magnesium balance in ambulatory care: the Donmag Study
(abst.). Magnes. Res. 8, (Supp.
1): 44-47.
35. Verri, A.P., Christina, S., Sandrini, G. & Nappi, G.
(1995) Psychiatric comorbidity in neuronal hyperexcitability
syndrome (abst.) Magnes. Res.
8,(Supp.1) 73.
36. Sandrini G., Verri, A.P., Costa, A., Musicco, M. &
Nappi, G. (1996): A clinical-epidemiological approach to the
nosography of the neuronal hyperexcitability syndrome. In:
Current research in magnesium, eds M.J. Halpern & J.
Durlach: pp. 155-160, London: John Libbey.
37. Lichodziejewska, B., Klos, J., Rezler, J., Grudska, K.,
Dlujniewska, M., Budaj, A. & Ceremuzynski, L. (1997):
Clinical symptoms of mitral valve prolapse are related to
hypomagnesemia and attenuated by magnesium supplementation.
Am. J. Cardiol. 79, 768-772.
38. Seelig, M. (1997): Might patients with the chronic fatigue
syndrome have latent tetany of magnesium deficiency? J. Am.
Coll. Nutr. 16, (in press).
39. Durlach, J. (1989): Recommended dietary amounts of
magnesium: Mg RDA. Magnes. Res. 2,
195-203.
40. Maertens De Noordhout, B., Henrotte, J.G. &
Franchimont, P. (1987): Latent tetany, magnesium and HLA tissue
antigens. Magnes. Bull. 9,
118-121.
41. Santarromana, M., Delepierre, M., Feray, J.C., Franck, G.,
Garay, R. & Henrotte, J.G. (1989): Correlation between total
and free magnesium levels in human red blood cells. Influence of
HLA antigens. Magnes. Res. 2,
281-283.
42. Henrotte, J.G. (1993): Genetic regulation of cellular
magnesium content. In: Magnesium and the cell. ed, N.J.
Birch. pp. 177-195. London, Boston: Academic Press.
43. Durlach, J., Poenaru, S., Rouhani, S., Bara, M. &
Guiet-Bara A. (1987): The control of central neural
hyperexcitability in magnesium deficiency. In: Nutrients and
Brain Function, ed. W.B. Essman. pp. 48-71. Basel, New York:
Karger.
44. Durlach, J. (1995): Editorial Policy of Magnesium
Research: general considerations on the quality criteria for
biomedical papers. Some complementary guidelines for the
contributors of Magnesium Research. Magnes. Res.
8, 191-206.
45. Bac, P., Pages, N., Herrenknecht, C., Dewulf, C., Binet,
P. & Durlach, J. (1994): Effect of various serotoninergically
induced manipulations on audiogenic seizures in
magnesium-deficient mice. Magnes. Res.
7, 107-115.
46. Mazur, A., Gueux, E., Remesy, C., Demigne, C. &
Rayssiguier, Y. (1989): Plasma and red blood cell magnesium
concentrations in Zucker rats: influence of a high fibre diet.
Magnes. Res. 2, 189-192.
47. Henrotte, J.G., Franck, G., Santarromana, M., Leloup, P.,
Motta, R., Biozzi, G. & Mouton, D. (1988): Selection of 2
lines of mice for high and low blood cell magnesium
concentrations called MGH (high) and MGL (low). Mouse News
Lett. 81, 84-85.
48. Henrotte, J.G., Aymard, N., Leyris, A., Monier, C.,
Frances, H. & Boulu, R.G. (1993): Brain weight and
noradrenaline content in mice selected for low (MGL) and high
(MGH) blood magnesium. Magnes. Res. 6,
21-22.
49. Aymard, N., Leyris, A., Monier, C., Frances, H., Boulu,
R.G. & Henrotte, J.G. (1995): Brain catecholamines, serotonin
and their metabolites in mice selected for low (MGL) and high
(MGH) blood magnesium levels. Magnes. Res.
8, 5-9.
50. Vink, R., Mcintosh, T.K., Demediuk, P. & Faden, A.I.
(1987): Decrease of total and free magnesium concentration
following traumatic brain injury in rats. Biochem.
Biophys. Res. Commun. 149, 594-599.
51. Vink, R., Mcintosh, T.K., Demediuk, P. Weiner, M.W. &
Faden, A.I. (1988): Decline in intracellular free Mg is
associated with irreversible injury after brain trauma. J.
Biol. Chem. 263, 757-761.
52. Vink, R. & Mcintosh, T.K. (1990): Pharmacological and
physiological effects of magnesium on experimental brain injury.
Magnes. Res. 3, 163-169.
53. Vink, R., Heath, D.L. & Mcintosh, T.K. (1996): Acute
and prolonged alterations in brain free magnesium following fluid
percussion-induced brain trauma in rats. J. Neurochem.
66, 2477-2483.
54. Mcintosh, T.K. Smith, D.H., Meaney, D.F., Kotapka, M.J.,
Gennarelli, P.A. & Graham, D.I. (1996): Neuropathological
sequelae of traumatic brain injury: relationship to neurochemical
and biomechanical mechanisms. Lab. Invest.
74, 315-342.
55. Suzuki, M., Nishina, M., Endo, M., Matsushita, K.,
Tetsuka, M., Shima, K. & Okuyama, S. (1997): Decrease in
cerebral free magnesium concentration following closed head
injury and effects of VA-045 in rats. Gen. Pharmac.
28, 119-121.
56. Cernak, I., Radosevic, P., Malicevik, Z. & Savic, J.
(1995): Experimental magnesium depletion in adult rabbits caused
by blast overpressure. Magnes. Res. 8,
249-259.
57. Mitani, K. (1992): Relationship between neurological
diseases due to Al load, especially amyotrophic lateral sclerosis
and magnesium status. Magnes. Res.
5, 03-213.
58. Yasui, M., Ota, K. & Garruto, R.M. [1995): Effects of
Ca-deficient diets on manganese deposition in the central nervous
system and bones of rats. Neurotoxicology
16, 511-517.
59. Yasui, M., Ota, K. & Yoshida, M. (1997): Effects of
low Ca and Mg dietary intake on the central nervous system
tissues of rats and Ca-Mg related disorder in the Kii peninsula
of Japan. Magnes. Res. 10, (in
press).
60. Bac, P., Herrenknecht, C., Binet, P. & Durlach, J.
(1993): Audiogenic seizures in magnesium-deficient mice: effects
of magnesium pyrrolidone-2-carboxylate, magnesium
acetyltaurinate, magnesium chloride and vitamine B-6. Magnes.
Res. 6, 11-19.
61. Bac, P., Pages, N., Herrenknecht, C. & Teste, J.F.
(1995): Inhibition of mouse killing behaviour in magnesium
deficient rats: effect of pharmacological doses of Mg pidolate,
Mg aspartate, Mg lactate, Mg gluconate and Mg chloride.
Magnes. Res. 8, 37-45.
62. Dupont, C. & Bac, P. (1996): Action of magnesium
acetyltaurinate on two models of magnesium depletion in rats and
mice (abst.). Magnes. Res. 9,
239-240.
63. Bac, P., Herrenknecht, C., Pages, N., Dupont, C. &
Durlach, J. (1996): Reversible model of magnesium depletion
induced by systemic kainic acid injection in magnesium deficient
rats: I. Comparative study of various magnesium salts.
Magnes. Res. 9, 281-292.
64. Poenaru, L., Crosnier, J.M., Manicom, R., Poenaru, S.,
Durlach, J., Rouhani, S., Rayssiguier, Y., Emmanouilidis, E.,
Gueux, E., Nkanga, W. & Soulairac, A. (1991): Regional
distribution of magnesium in the cerebral tissue in normal and in
magnesium deficient rat (abst.). Magnes. Res.
4, 249.
65. Lerma, A., Planells, E., Aranda, P. & Llopis, J.
(1993): Evolution of magnesium deficiency in rats. Ann.
Nutr. Metabot. 37, 210-217.
66. Delorme, O., Bourdin, H., Viel, J.F., Simon Rigaud, M.L.
& Kantelip, J.P. (1992). Spectral analysis of
electroencephalography data in athletes with low erythrocyte
magnesium. Magnes. Res. 5,
261-264.
67. Penland, J.G. (1995): Quantitative analysis of EEG effects
following experimental marginal magnesium and boron deprivation.
Magnes. Res. 8, 341-358.
68. Poenaru, S., Durlach, J., Rouhani, S. Rayssiguier, Y.,
Iovino, M. & Reba, A. (1983): Etude
électrophysiologique de la carence magnésique du
rat. Magnesium 2, 299-312.
69. Depoortere, H., Francon, D. & Llopis, J. (1993):
Effects of a magnesium deficient diet on sleep in rats.
Neuropsychobiology 27, 237-245.
70. Duc, M., Duc, M.L., Faure, G., Grandelaude, X. & Mur,
M.M. (1980): Magnesium blood levels and nervous hyperexcitability
syndrome. In: Magnesium in health and disease. eds M.
Cantin & M.S. Seelig, pp. 777-784. New York: Spectrum.
71. Mazotta, G., Sarchielli, P., Alberti, A. & Gallai, V.
(1996): Electromyographic ischemic test and intra-cellular and
extra-cellular magnesium concentration in migraine and
tension-type headache. Headache 36,
257-261.
72. Swanson, D.R. (1988): Migraine and magnesium: eleven
neglected connections. Perspect. Biol. Med.
31, 526-557.
73. Ramadan, N.M., Halvorson, H., Vande-Linde, A.M.Q., Levine,
S.R., Helpern, J.A. & Welch, K.M. (1989): Low brain magnesium
in migraine, Headache 29, 590-593.
74. Thomas, J., Thomas, E. & Tomb, E. (1992): Preliminary
communication: serum and erythrocyte concentrations and migraine.
Magnes. Res. 5, 127-130.
75. Sarchielli, P., Coata, G., Firenze, C., Morucci, P.,
Abbritti, G. & Gallai, V. (1992): Serum and salivary
magnesium levels in migraine and in tension-type headache. Result
in a group of adult patients. Cephalalgia
13, 94-98.
76. Mauskop, A., Altura, B.T., Cracco, R.Q. & Altura, B.M
(1993): Deficiency in serum ionized magnesium, but not total
magnesium in patients with migraines: possible role of ionized
Ca/ionized Mg ratio. Headache 33,
135-138.
77. Gallai, V., Sarchielli, P., Morucci, P. & Abbritti, G.
(1994): Magnesium content of mononuclear blood cells in migraine
patients. Headache 34, 160-165.
78. Mauskop, A., Altura, B.T., Cracco, R.Q. & Altura, B.M.
(1994): Chronic daily headache. One disease or two. Diagnostic
role of serum ionized magnesium. Cephalalgia
14, 24-28.
79. Smeets, M.C., Vernooy, C.B., Souverijn, J.H.M. &
Ferrari, M.D. (1994): Intracellular and plasma magnesium in
familial hemiplegic migraine and migraine with and without aura.
Cephalalgia 14, 29-32.
80. Thomas, J., Tomb, E., Thomas, E. & Faure, G. (1994):
Migraine treatment by oral magnesium intake and correction of
buccofacial and cervical muscles as a side effect of mandibular
imbalance; Magnes. Res. 7, 123-127.
81. Saurian, S., Arnaldi, C., De Carlo, L., Arcudi, D.,
Mazzotta, D., Battistella, P.A., Sartori, S. & Abbasciano, J.
(1995): Serum and red blood cell magnesium levels in juvenile
migraine patients. Headache 35,
14-16.
82. Welch, K.M.A. & Ramadan, N.M. (1995): Mitochondria,
magnesium and migraine. J. Neurol. Sci.
134, 9-14.
83. Evers, S., Suhr, B. & Staschewski, F. (1996):
Magnesium in the long term treatment of migraine: an open,
prospective and non controlled study. In: Current research in
magnesium, eds M.J. Halpern & J. Durlach, pp. 359-362.
London: John Libbey.
84. Thomas, J. Sebille, S., Millot, J.M., Arnaud, M.,
Delabroise, A.M., Millart, H., Thomas, E., Tomb, E. &
Manfait, M. (1996): Intracellular free and total magnesium in
lymphocyte cells from migraine patients : effect of Mg
rich-mineral water intake (abst.). Magnes. Res.
9, 243.
85. Cox, I.M., Campbell, M.J. & Dowson, D. (1991): Red
blood cell magnesium and chronic fatigue syndrome.
Lancet 337, 757-760.
86. Durlach, J. (1992): Chronic fatigue syndrome and chronic
primary magnesium deficiency. Magnes. Res.
5, 68.
87. Moorkens, G., Manuely, Deenoy, B., Vertommen, J., Meludu,
S., Noe, M. & De Leeuw, I. (1996): Magnesium deficiency in a
sample of the Belgian population presenting with symptoms
suggestive of chronic fatigue syndrome (abst.). Magnes.
Res. 9, 242.
88. Kirov, G.K., Birch, N.J., Steadman, P. & Ramsey, R.G.
(1994): Plasma magnesium levels in a population of psychiatric
patients: correlation with symptoms. Neuropsychobiology
30, 73-78.
89. Tuchendria, E., Pacamaru, I., Paradopol, V., Duda, R.,
Laszlo, L. & Rusu, L. (1996): Magnesium deficit: a possible
causal factor of some neurotic tendencies in elderly persons
(abst.). Magnes. Res. 9, 232-233.
90. Durlach, J. (1990): Magnesium depletion and Alzheimer
disease. Magnes. Res. 3, 217-218.
91. Romano, T.J. & Stiller, J.W. (1994): Magnesium
deficiency in fibromyalgia syndrome. J. Nutr.
Med. 4, 165-167.
92. Clauw, D.J., Ward, K., Wilson, B., Katz, P. & Rajan,
S.S. (1994): Magnesium deficiency in the eosinophilia-myalgia
syndrome: report of clinical and biochemical improvement with
repletion. Arthritis Rheumatism 37,
1331-1334.
93. Eisinger, J., Plantamura, A., Marie, P.A. & Ayavou, T.
(1994): Selenium and magnesium status in fibromyalgia.
Magnes. Res. 7, 285-288.
94. Eisinger, J., Zakarian, H., Pouly, E., Plantamura, A.
& Ayavou, T. (1996): Protein peroxidations, magnesium
deficiency and fibromyalgia. Magnes. Res.
9, 313-316.
95. Popoviciu, L.B.A., Delast-Popoviciu, D., Alexandrescu, A.,
Petrutiu, S. & Bagathal, I. (1993): Clinical, EEG,
electromyographic and polysomographic studies in restless legs
syndrome. Romanian J. Neurol. Psychiatry
31, 55-61.
96. Myrdal, U., Leppert, J., Edvinsson, L., Ekman, R., Hedner,
T., Nilson, H. & Ringqvist, I. (1994): MgSO4
infusion decreases circulating calcitonin gene-related peptide
(CGRP) in women with primary Raynaud's phenomenon. Clin.
Physiol. 14, 539-546.
97. Durlach, J. (1995): Magnesium depletion, magnesium
deficiency and asthma. Magnes. Res.
8, 403-405.
98. Rylander, R., Dahlberg, C. &, Rubenowitz, E. (1997):
Magnesium supplementation decreases airway responsiveness among
hyperreactive subjects. Magnes. Bull.
19, 4-6.
99. Ismagilov, M.F., Tananov, A.T. & Kurmyshkin, A.A.
(1992): HLA antigens in persons predisposed to frequent Sympatho
Adrenal Paroxysm. Zh. Nevropatol. Psikhiatr.
Imeni Korsakova 92, 35-37.
100. Cippola, C., Occhionero, T., Orciari, P., Lugo, G. &
D'antvono, G. (1990): Magnesium pidolate in the treatment of
seasonal allergic rhinitis. Magnes. Res.
3, 109-112.
101. Mccoy, H. & Kenney, M.A. (1992): Magnesium and immune
function: recent findings. Magnes. Res.
5, 281-294.
102. Mansmann Jr, H.C. (1995): Concurrent normomagnesemic
magnesium deficiency in an allergy practice (abst.).
8, (Supp. 1) 49-50.
103. McCoy, H. & Kenney, M.A. (1996): Interactions between
magnesium and vitamin D : possible implications in the immune
system. Magnes. Res. 9, 185-203.
104. Kozielec, T., Starobrat-Hermelin, B. & Kotkowski, L.
(1996): The effect of magnesium deficiency on children with
attention deficit hyperactivity disorder (ADHD). (abst.).
Magnes. Res. 9, 244.
105. Schmidt, M.E., Kruesi, M.J.P., Elia, J., Borcherding,
B.G., Elin, R.J., Hosseini, J.M., Mcfarlin, K.E. & Hamburger,
S. (1994): Effect of dextroamphetamine and methylphenidate on
calcium and magnesium concentration in hyperactive boys.
Psychiatry Res. 54, 199-210.
106. Ratzman, G.W. (1995): Vegetative functional disorders,
tetanic disposition and disturbances of magnesium homeostasis in
children and adolescents. (abst.) Magnes. Bull.
17, 139.
107. Schimatschek, H.F., Classen, H.G., Baerlocher, K., Thoni,
H. & Wendt, B. (1995): Proof of an oral magnesium
supplementation, in hypomagnesemic children with functional
neurovegetative disorders, results of an ambulatory, multicentric
randomized, activeplacebo controlled double blind trial. (abst.).
Magnes. Bull. 17, 139.
108. Schleir, E., Schelhorn, D. & Groh, F. (1991):
Biochemical studies in stuttering children. Otolaryngol.
Pol. 45, 141-144.
109. Durlach, J., Durlach, V., Rayssiguier, Y., Ricquier, D.,
Goubern, M., Bertin, R., Bara, M., Guiet-Bara, A., Olive, G.
& Mettey, R. (1991): Magnesium and thermo-regulation I.
Newborn and infant. Is Sudden Death Syndrome a
magnesium-dependent disease of the transition from chemical to
physical thermo-regulation? 4, 137-152.
110. Caddell, J.L. (1992): Hypothesis: new concepts concerning
the pathophysiology of the Sudden Infant Death Syndrome due to
magnesium deficiency shock. Magnes. Res.
5, 165-172.
111. Caddell, J.L. (1993): Hypothesis : Possible links between
the respiratory distress syndrome of the premature neonate, the
Sudden Infant Death Syndrome and magnesium deficiency shock.
Magnes. Res. 6, 25-32.
112. Lukacsi, L., Lintner, F., Gimes, G., Zsolnai, B. &
Somogyi, J. (1993): Magnesium content of human myometrium and
placenta during various stages of gestation and of different body
fluids at term. Magnes. Res. 6,
47-52.
113. Durlach, J. (1993): Death from infancy to older age and
marginal maternal magnesium deficiency. How long should the
follow up of the consequences of under nutrition in pregnancy be
continued? Magnes. Res. 6, 297-298.
114. Bubeck, J., Haussecker, H., Disch, G., Spatling, L. &
Classen, H.G. (1994): Potentiation of
magnesium-deficiency-induced foetotoxicity by concomitant iron
deficiency and its prevention by adequate supply via drinking
water. Magnes. Res. 7,
245-254.
115. Durlach, J. & Durlach, V. (1995): Combien de temps
faut-il suivre les conséquences d'un trouble nutritionnel
gravidique? ou... des protéines, du magnésium et de
la mort. Med. Et Nutr. 31, 38-39.
116. Caddell, J.L. (1995): Hypothesis: the possible role of
magnesium and copper deficiency in retinopathy of prematurity.
Magnes. Res., 8, 261-270.
117. Caddell, J.L. (1996): A review of evidence for a role of
magnesium and possibly copper deficiency in necrotizing
enterocolitis. Magnes. Res. 9,
55-66.
118. Caddell, J.L. (1996): Evidence for magnesium deficiency
in the pathogenesis of bronchopulmonary dysplasia (BPS).
Magnes. Res. 9, 205-216.
119. Costello, R.B. & Moser-Veillon, P.B. (1992): A review
of magnesium intake in the elderly. A cause for concern?
Magnes. Res. 5, 61-68. 11
120. Rayssiguier, Y., Durlach, J., Gueux, E., Rock, E. &
Mazur, A. (1993): Magnesium and ageing. I. Experimental data,
importance of oxidative damage. Magnes. Res.
6, 369-378.
121. Durlach, J., Durlach, V., Bac, P., Rayssiguier, Y., Bara,
M. & Guiet-Bara, A. (1993): Magnesium and ageing. II.
Clinical data: aetiological mechanisms and pathophysiological
consequences of magnesium deficit in the elderly. Magnes.
Res. 6, 379-394.
122. Simekova, A., Zamrazil, V. & Cerovska, J. (1996):
Morning magnesiuria: relation to age and sex. Magnes.
Res. 9, 41-45.
123. Singh, R.B., Niaz, M.A . Ghosh, S. & Rastogi, V.
(1996): Epidemiological study of magnesium status and risk of
coronary artery disease in elderly rural and urban populations of
North India. Magnes. Res. 9,
165-172.
124. Singh, R.B., Rastogi, V., Singh, R., Niaz, M.A.,
Srivastav, S., Aslam, M. & Singh, N.K. (1996): Magnesium and
antioxidant vitamin status and risk of complication of ageing in
an elderly urban population. Magnes. Res.
9, 299-306.
125. Boggio, V., Guilland, J.G., Moreau, D., Durlach, J. &
Klepping, J. (1986): Dietary magnesium intake among male athletes
in France. (abst.). Magnes. Bull. 8,
275.
126. Rayssiguier, Y., Guezennec, C.Y. & Durlach, J.
(1990): New experimental and clinical data on the relationship
between magnesium and sport. Magnes. Res.
3, 93-101.
127. Ruddel, H., Werner, C. & Ising, H. (1990): Impact of
magnesium supplementation on performance data in young swimmers.
Magnes. Res. 3, 103-107.
128. Stendig-Lindberg, G., Wacker, W.E.C. & Shapiro, Y.
(1991): Long term effects of peak of strenuous effort on serum
magnesium, lipids and blood sugar in apparently healthy young
men. Magnes. Res. 4,
59-65.
129. Laires, M.J. & Alves, F. (1991): Changes in plasma,
erythrocyte and urinary magnesium with prolonged swimming
exercise. Magnes. Res. 4, 119-122.
130. Stendig-Lindberg, G. (1992): Is physical working capacity
determined by optimal magnesium concentration? J. Basic
Clin. Physiol. Pharmacol. 3,
139-151.
131. Stendig-Lindberg, G. (1992): Sudden death of athletes: is
it due to long term changes in serum magnesium, lipids and blood
sugars J. Basic Clin. Physial. Pharmocol.
3, 153-164.
132. Cordova, A., Escanero, J.F. & Gimenez, M. (1992):
Magnesium distribution in rats after maximal exercise in air and
under hypoxia conditions. Magnes. Res.
5, 23-27.
133. Singh, R.B., Rastogi, S.S., Ghosh, S., Niaz, M.A. &
Singh, N.K. (1992): The diet and moderate exercise trial (Damet):
results after 24 weeks. Acta Cardiol.
47, 543-557.
134. Madsen, K., Pedersen, P.K., Djurhuus, M.S. &
Klitgaard, N.A. (1993): Effects of detraining on endurance
capacity and metabolic changes during prolonged exhaustive
exercise. J. Appl. Physiol. 75,
1444-1451.
135. Laires, M.J., Madeira, F., Sergio, J., Colaco, C., Vaz,
C., Felisberto, G.M., Neto, I., Breitenfeld, L., Bicho, M. &
Manso, C. (1993): Preliminary study of the relationship between
plasma and erythrocyte magnesium variations and some circulating
prooxidant and antioxidant indices in a standardised physical
effort. Magnes. Res. 6, 233-238.
136. Laires, M.J. (1996): Magnesium and sport. In: Current
research in magnesium. eds M.J. Halpern & J. Durlach,
pp. 221-225. London: John Libbey.
137. Monteiro, C.P., Palmeira, A., Felisberto, G.M., Vaz, C.,
Rodrigues, A., Barata, J. & Laires, M.J. (1996): Magnesium,
calcium, trace elements and lipid profile in trained volleyball
players: influence of training. In: Current research in
magnesium eds M.J. Halpern & J. Durlach, pp; 231-235.
London: John Libbey.
138. Pereira, D. Laires, M.J., Monteiro, C.P., Rabacal C.,
Ribeiro, H., Felisberto, G., Mendonca, C.: Vaz, C., Nuno, L.,
Dias, M., Carvalho, E., Afonso, J.S. & Fernandes, J.S.
(1996): Oral magnesium supplementation in heavily trained
football players: impact on exercise capacity and
lipoperoxidation. In: Current research in magnesium. eds
M.J. Halpern & J. Durlach, pp 237-241. London: John
Libbey.
139. Madsen, K., Ertbjerg, P., Djurhuus, M.S. & Pedersen,
P.K. (1996): Calcium content and respiratory control index of
skeletal muscle mitochondria during exercise and recovery.
Am. J. Physiol. 271, E 1044-E 1050.
140. Durlach, J. (1988) Chronic alcoholism. In: Magnesium
in clinical practice, ed. J. Durlach, pp. 120-133.
London- John Libbey.
141. Gullestad, J., Dolva, L. O., Soyland, E., Manger, A.T.,
Falch, D., Veirod, M.B. & Kjekshus, J. (1991): Effects of
oral magnesium treatment in chronic alcoholics In: Magnesium
in clinical practice, ed. J. Durlach, pp. 405-409. London:
John Libbey.
142. Gullestad, L., Olva, L.O., Manger, A.T., Falch, D. &
Kjekshus, J. (1992): Oral magnesium supplementation improves
metabolic variables and muscle strength in alcoholics.
Alcoholism Clin. Exp. Res. 16,
986-990.
I43. Stendig-Lindberg, G. (1974): Hypomagnesemia in alcohol
encephalopathies. Acta Psychiatr. Scand.
50, 465-480.
144. Stendig-Lindberg, G. & Rudy, N. (1980): Stepwise
regression analysis of an intensive 1- year study of delirium
tremens. Acta Psychiatr. Scand. 62,
273-297.
145. Durlach, J. (1988): Magnesium deficit in diabetes
mellitus. In: Magnesium in clinical practice,
ed. J. Durlach, pp. 156-169. London: John Libbey.
146. Joslyn, S., Lynch, C., Wallace, R., Olson, D. & Van
Hoesen, C. (1990): Relation between diabetes mellitus mortality
rates and drinking water magnesium levels in Iowa. Magnes.
Trace Elem. 8, 94-100.
147. Gullestad, L. Jacobsen, T. & Dolva, L.O. (1994)-
Effects of Mg treatment on glycemic control and metabolic
parameters in NIDDM patients. Diabetes Care
17, 460-461.
148. de Valk, H.W., Verkaaik, R., Van Rijn, H.J.M., Geerdink,
R.A., Haalboom, J.R.E. & Struyvenberg, A. (1996): 3 months
oral Mg supplementation in insulin-requiring patients with NID
(type 2) diabetes mellitus. In: Current Res. in Mg. eds
M.J. Halpern and J. Durlach, pp. 117-119. London: John
Libbey.
149. Durlach, J., Bara, M. & Guiet-Bara, A. (1990):
Magnesium and its relationship to oncology. In: Metal ions
and biological systems, vol. 26. Compendium on
magnesium and its role in biology, nutrition and physiology, ed.
H. Sigel, pp. 546-578. New York: Marcel Dekker.
150. Durlach, J., Guiet-Bara, A., Bara, M., Rayssiguier, Y.
& Collery, P (1990): Cancer and magnesium status. In:
Metal ions in biology and medicine. eds P. Collery, L.A.
Poirier, M. Manfait & J.C. Etienne, pp. 40-44. London, Paris:
John Libbey-Eurotext.
151. Gonella, M. & Calabrese, G. (1989): Magnesium status
in chronically haemodialyzed patients: the role of dialysate
magnesium concentrations. Magnes. Res.
2, 259-266.
152. Widmer, J., Stella, N., Raffin, Y., Bovier, P., Gaillard,
J.M., Hilleret, H. & Tissot, R. (1993): Blood Mg, K, Na, Ca
and cortisol in drug free depressed patients. Magnes.
Res. 6, 33-41.
153. Kisters, K., Spieker, C., Tepel, M., Wenserski, F. &
Zidek, W. (1995): Plasma, systolic and membrane Mg content in
renal insufficiency. Magnes. Res.
8, 167-172.
154.7 Cordova, A. (1995): Variations of serum magnesium and
zinc after surgery and postoperative fatigue. Magnes.
Res. 8, 367-372.
155. Widmer, J., Henrotte, J.G., Raffin, Y., Mouthon, D. &
Bovier, P. (1996): Relationship with hypoexcitability syndrome
and plasma and erythrocyte magnesium in several depressed
patients (abst.) Magnes. Res. 9,
245.
This page was first uploaded to The Magnesium Web Site on
March 2, 1998
http://www.mgwater.com/











