Magnesium 4: 5-15 (1985)
Magnesium Level in Drinking Water and Cardiovascular Risk
Factor: A Hypothesis
J. Durlacha, M. Barab, A.
Guiet-Barab
aCHU Cochin-Port-Royal et bLaboratoire
de Biologie de la Reproduction,
Université Pierre-et-Marie-Curie, Paris, France
Key Words. Magnesium · Drinking water
· Cardiovascular mortality
Abstract. Water hardness can no longer be
considered as the most reliable ‘water factor’ with
regard to the cardiovascular risk observed in epidemiologic
studies. Only two out of three studies have shown a reverse
correlation between cardiovascular mortality and water
hardness. But studies carried out on the water Mg level alone,
as opposed to those on water hardness (Ca + Mg) have all shown
a reverse correlation between cardiovascular mortality and the
Mg level.
In developed countries, the Mg intake is often marginal and
the Mg intake coming from drinking water represents the critical
factor through which the intake is deficient or satisfactory.
Thus, Mg deficiency, either experimental or in man facilitates
cardiovascular pathology.
The importance of the Mg intake in drinking water is both
quantitative and qualitative. Water containing Mg is better and
more quickly absorbed than dietary Mg. This particular
availability might help to understand why an adequate water Mg
level may determine a better state of health, even without any Mg
deficiency. Epidemiological data in man and experimental data in
rats have demonstrated that the intake of water containing a
sufficient amount of Mg may prevent arterial hypertension and
correlated ionic and nervous disturbances.
Indirectly the water Mg level also interferes in the leakage
of food-borne Mg during cooking. There is an inverse correlation
between the Mg loss in the cooked food and the Mg level of the
cooking water itself. Mg appears to be an antagonist of noxious
polluting agents (e.g. in the human amnion, Mg is a competitive
inhibitor of Pb and Cd). It is not advisable to enrich water in
Mg in the course of the processing since its corrosivity index
would also increase. The best pathway is probably to neutralize
corrosive water by filtration on calibrated grains of
earth-alkaline metal (Neutralite or Magno or Akdolit) to ensure
the highest possible Mg/Ca ratio, with the best anticorrosive
power.
Introduction
In 1957, Kobayashi [58] pointed out the possible
relationship between the composition of drinking water and
cardiovascular diseases after observing a geographical
correlation between stroke mortality and the water acidity of the
rivers. Schroeder [96] statistically checked the
validity, of the Japanese data, and then examined the
epidemiological implications of this phenomenon in the United
States. He suggested an inverse correlation between various types
of heart diseases and total drinking water hardness: drinking
soft water increases the cardiovascular risk which is
reciprocally reduced by hard water consumption.
In all continents, numerous studies have been devoted to this
inverse correlation through a large number of statistical units
of.observations (states, towns, districts) diversification of the
causes of mortality (general, cardiac, vascular) and
multiplication of observed parameters (analytical data on water,
climatic and geological factors) [27, 49, 54, 71-78, 86]. The
inverse correlation has been confirmed in the majority of the
studies and, in particular, when the geographical scale of the
epidemiological study is larger, i.e. when the cultural, climatic
and professional parameters vary widely. However, the inverse
relationship between the hardness of the water and cardiovascular
mortality is not always found [26, 27, 51]. Consequently, the
water parameter cannot be considered a major risk since it may be
completely missing; but studies on the water factor have shown
the frequency of the relationship between cardiovascular diseases
and drinking water. Other etiological and atmospheric cofactors,
associated with hardness (i.e. pluviosity [49] or
salt-consumption [28]) might have possible roles, but the main
question concerns the relationship between drinking water and a
satisfactory condition of the cardiovascular apparatus. Two types
of research have attempted to determine either the cardiovascular
protecting factors in protective water, or the cardiovascular
toxic factors in noxious water. These water factors are only to
be taken into consideration when they represent a notable part of
the recommended dietary allowances or qualitatively, if they
constitute a higher bioavailability intake.
Accessory Protective Factors in Drinking Water
A significant inverse correlation has been irregularly found
between cardiovascular mortality and natural water elements:
K+ [98], Ca2+ [98], Li+ [71],
CO3H- [98], F- [67],
I- [77, 78], Ni [71], Cr, Fe, Zn, Cu, Se [41, 77, 78,
80], perhaps Va [44, 96], Si [99] and As [89]. It is obvious that
if the importance of dietary K intake (particularly the K/Na
ratio [69, 85, 105] is critical, the quantitative part of water K
is rarely important. This observation is identical with that made
on Ca. Its dietary intake has an important cardiovascular
protective role [10, 12, 68, 89, 94], but Ca in drinking water,
which is often poorly assimilated, represents a negligible
element of the Ca intake. The importance of the Ca level in
drinking water can be presented to the consumer as an
anticorrosive substrate of the protective coating deposited on
the pipes by nonaggressive waters. The inverse correlation
between Li+ and mortality is low and becomes inverse
when the partial correlations are calculated [71]. Nevertheless,
the lowered bicarbonate and carbonate levels are essential
elements of the corrosivity [7, 8, 99]. The water contribution of
various trace elements, the minimum requirements of which are
known (F, I, Cr, Fe, Zn, Cu), may be important, but it is
difficult to appreciate this contribution when the requirements
have not yet been defined (As, Si ...).
Magnesium: The Major Protective Water Factor
Of all the factors studied in drinking water, the highest
inverse correlation has been observed between Mg and
cardiovascular mortality [2-4, 54, 71-76 100, 108a, 109]
Moreover, in Canada, the myocardial level is significantly lower
in soft water districts than in hard-water districts. The same is
found in the hearts of the subjects who died from sudden heart
attacks or myocardial infarcts (even outside necrosed tissue
areas where the leak also depends on tissue lysis [3, 5, 6,
23-25, 71-75, 106]). Quantitatively, the water Mg contribution
may represent the amount of Mg required to bring an insufficient
dietary Mg level to a correct level [3, 42, 54, 71-75, 77, 78,
102, 109]. In fact, in developed countries, the adult Mg intake
is marginal as to the ‘recommendable’dietary
allowance [6 mg/kg/day; 36, 100, 101] and even to the slightly
lower recommended dietary allowance (5 mg/kg/day) proposed by the
US National Academy of Sciences in adults. The inadequacy of the
Mg intake is even worse in anabolic phases such as growth,
pregnancy, lactation...
According to its Mg content (from 10 to 100 mg/l), 2 liters of
water. (drinking water, tea, coffee, soups) increase the Mg
intake from 20 to 200 mg/day. A higher water Mg intake is even
observed with some bottled mineral waters, i.e. Vittel
Hépar: 130 mg/l [83].
The Mg level of cooking water also interferes in the Mg
dietary intake. A nutrient in the cooking water, always loses an
important part of its Mg [95], but the Mg loss is lower when the
food is cooked in a water with high Mg content. There is an
inverse correlation between the Mg loss in the cooked food and
the Mg level of the cooking water [28, 36, 47, 77]. Nowadays,
housewives often cook with softened water. They would do better
to use unsoftened drinking water.
Thus, quantitatively, the magnesium of drinking water
represents an appreciable part of the dietary intake. As a risk
index, the Mg level in water is more satisfactory than hardness.
According to a number of reports its participation in hardness
ranges from 10 to 89% [75, 76]. One should distinguish between
hard water content in Great Britain [74] France [29, 108],
uncorrelated with cardiovascular mortality and the US hard waters
with high Mg contents [75, 76] and reversely correlated with
cardiac diseases [46]. It is to be hoped that in water, as well
as in-the overall diet [47], the Mg/Ca ratio is not too low. As a
matter of fact, in France and Great Britain, the Mg/Ca ratio
amounts on the average to a. few hundredths, while in the US, it
ranges from 1 to 5.
The water Mg intake may quantitatively represent the critical
contribution allowing the control of an Mg deficiency whose
physiological effects on the cardiovascular apparatus are
perfectly well known [2-4, 11, 100].
But, the contribution of Mg in drinking water is also
interesting qualitatively.
Studies conducted by Lowik et al. [66] and
Binnerts et al. [13] with diets. poor, normal and rich
in Mg show that with an absolute. equivalent Mg intake, Mg in
drinking water is much better absorbed than dietary Mg. This
particular bioavailability of water Mg can be proven
experimentally. The mortality rate of mice in which Mg deficiency
had been induced by a diet with a very low Mg content could be
controlled by the mere intake of water containing 30 mg Mg/l [54,
109]. This Mg level is found in various hard waters of the
distribution system. The boiling of water, decreases the Ca
level, but has little effect on the Mg level.
Breakfast should provide an intake of water Mg since the Mg
requirements increase when people resume their activities.
Suppressing breakfast and/or the coffee break induces massive
reduction of magnesuria [13, 66].
Accordingly, the urinary Mg/Ca ratio is higher than it is in
the ingested water [61, 62]
These particular absorption qualities of water Mg [65] suggest
an interpretation of the investigations of Novikov et
al. [84]. The study of various cardiovascular, nervous and, ionic
parameters in, Siberian populations, comparable in all respects
except for the hardness of their drinking water, shows an inverse
correlation between soft water with a low Mg content and arterial
hypertension as well as K-Na, P-Ca and neuroendocrine alterations
which may cause vascular pathology.
Rats were administered a balanced diet, namely with an
adequate Mg level: blood and tissue Mg was normal in all these
animals. But the Mg and Ca levels of their drinking water were
different in each group. The results showed similar correlations
between the Mg water level and vascular impairments with similar
ionic and neuroendocrine disorders in the rats and the Siberian
populations. This suggests that an insufficient Mg level in
drinking water can induce hypertension together with
neuroendocrine and humoral disorders in both animals and man.
Recently, Altura and Altura [3a] have indeed demonstrated that
alterations in dietary and waterborne Mg intake can produce
hypertension in rats; they found an inverse correlation between
serum Mg level and elevation in arterial blood pressure which was
linked to microvascular changes.
The effect of Mg contained in drinking water could be
explained by its particular bioavailability. In awakening
periods, when the metabolic Mg requirements are increased, an
adequate intake of quickly and well-absorbed water Mg would avoid
solicitation of the neuroendocrine controls of the Mg homeostasis
(vegetative, adrenal, thyroparathyroid and pancreatic [34, 35])
When the water Mg intake is too low the necessity of maintaining
the Mg homeostasis might induce, in the long run, a dysfunction
of these regulations . Since the diverse regulatory mechanisms of
Mg homeostasis are the same as those which control the K-Na and
P-Ca balance [34, 35], it is conceivable that their dysfunction
might induce nervous hyperexcitability and arterial
hypertension.
Drinking water containing Mg as a specific element of Mg
intake, qualitatively and quantitatively, is particularly
beneficial to the cardiovascular and nervous functions. This is
probably due to the fact that dietary Mg intake acts as an
antagonist of certain noxious factors of the water.
Noxious Factors of the Water: ‘Natural’ and
‘Polluting’ Factors; Advantage of the Ryznar
Corrosivity Index
‘Natural’ Elements: Na and Na/K Ratio
The noxious effects of Na on blood vessels have been known for
over 4,500 years [37]. The industrial civilization consumes too
much Na [81] and not enough K [41, 69, 81, 85, 93, 105]. Numerous
studies have considered Na as a major risk factor in drinking
water [14, 20, 21, 112]. In the majority of the epidemiological
studies [39], a direct correlation between hypertension and the
Na level in drinking water has been observed. However, this
relation is not consistent [39, 98]. It is obvious that the Na of
drinking water is not a major element of the Na intake, but it
can easily be modified. It is difficult to cut down by 50% the Na
intake which is provided in meat and dairy products! People are
more reluctant to reduce their usual consumption of salt. But one
should avoid the consumption and use of cooking water which is
very rich in Na such as artificially softened waters [39], whose
Na levels can be as high as 500 mg/l. It should be mentioned that
the use of soft waters seems to involve an increased consumption
of salt, as demonstrated by a direct correlation with natriuria
[28].
Water intake represents only a small part of the total Na
level and of the Na/K ratio in the general intake. Still, its
importance should not be overlooked in cases of specific
backgrounds such as hypertension and of consumption of soft
waters with high Na contents which result in increased
consumption of salt.
Corrosivity and ‘Polluting’ Metals
As early as 1974, Schroeder and Kraemer [99] thought
that the noxious cardiovascular effects of certain soft waters
might be due to the presence of toxic metals solubilized in the
geological layers or in the pipes.
Kobayashi [cited by Schroeder, 96] gives a
prominent position to the direct correlation between the
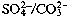 ratio-related
to the water acidity (X 3-5) and diseases such as hypertension
and strokes in Japan.
ratio-related
to the water acidity (X 3-5) and diseases such as hypertension
and strokes in Japan.
Water alkalinity (bicarbonate CO3H [99] and
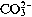 level [7,
8]) might be a protective factor. One should distinguish between
two physical factors of water: aggressivity towards Ca carbonate
and corrosivity to metals. A nonaggressive water may be
corrosive. These two notions can, however, be connected because
of the occurrence of a calcium deposit which protects the pipes
from corrosion. Corrosivity decreases together with aggressivity
[30, 56]. The Langelier index [63] determines whether a water is
encrusting (IL > 0) or aggressive (IL
< 0); a positive response of IL is a sign of
anti-aggressivity.
level [7,
8]) might be a protective factor. One should distinguish between
two physical factors of water: aggressivity towards Ca carbonate
and corrosivity to metals. A nonaggressive water may be
corrosive. These two notions can, however, be connected because
of the occurrence of a calcium deposit which protects the pipes
from corrosion. Corrosivity decreases together with aggressivity
[30, 56]. The Langelier index [63] determines whether a water is
encrusting (IL > 0) or aggressive (IL
< 0); a positive response of IL is a sign of
anti-aggressivity.
Ryznar [92] has proposed an empirical index
(IR), which appears to be more representative of the
corrosive or scaling characteristics of the water: IR
< 6: scaling water; 6 < IR < 7: balanced
water; IR > 7: corrosive water.
The Ryznar index should be systematically used in
epidemiological studies on the relationship between drinking
water and health. It is obvious that a very corrosive water can
be contaminated by metals classified into ‘essential’
elements and ‘toxic’ polluting factors. But this
classification is unsatisfactory since high levels of
‘essential’ elements become toxic and conversely low
levels of ‘toxic’ elements appear to be essential
[109]. For example, a negative correlation is found between
cardiovascular mortality and low levels of Fe, Cu, Cr, Mn, Zn, As
intake, but high levels of Ca [67, 70, 71, 88, 99, 102], Cr [71,
99], Mn [70, 71, 99], V [45], Zn (57), and As [111] have
polluting, toxic effects. The noxiousness of corrosive waters. is
mainly due to two toxic heavy metals: Pb and Cd [17, 18, 39,
102]. Water Pb does not have a geological origin. Unlike Cd, it
has a pseudocolloidal form which, in the course of further
processing, easily flocculates and is eventually absorbed. The
lead loading occurs in the distribution system [17, 18, 39, 102].
A short portion of lead water pipe, lead solders, stagnation in
the pipes (therefore high Pb levels in the early morning, heating
of the water, earth plugging of an electrical appliance) make the
problem even worse and can bring about elevated levels of Pb in
the water [32, 33, 114]. Attempts have been made to replace lead
pipes with galvanized iron pipes. However, Zn which is used for
galvanization always contains Cd, which is released when
corrosive water stagnates in the pipes [17, 18]. The use of
polyvinyl chloride (PVC) pipes does not always solve the water
pollution problem, as PVC may contain lead salts as stabilizing
agents and may contaminate the water [16].
The effects of water-polluting agents cumulated with those
from digestive and pulmonary sources are greatly to be feared,
because cation absorption is increased when the cations are water
soluble [109]. Besides, even low levels [59, 107] of Cd and Pb
have detrimental cumulative effects on the cardiovascular system
[80, 90], as they increase tissue Na and Ca levels and decrease
the K and Mg levels. These effects, which are comparable to those
of Mg deficiency, can be controlled by Mg intake. Indeed, Cd
intoxication decreases the Mg level in bone, teeth and soft
tissues [52, 53, 59, 113]. Conversely, Mg reduces Cd absorption
[109, 110] and is a competitive inhibitor of Cd in the isolated
amnion (unpublished data). Mg, partly, antagonizes the
carcinogenic effects of Cd [87] and antagonizes the uncoupling of
oxidative phosphorylation induced by Cd in hepatic mitochondria
but not in renal mitochondria [48]. The increase in Mg intake
reduces Pb retention and increases Pb excretion [79, 104]. But a
decrease of Pb absorption due to the described Mg intake [38] has
not been confirmed [109, 110], and is ancillary because the
increase in the urinary Pb level observed in the course of
parenteral Mg loading cannot be easily explained through this
mechanism [60]. Conversely, Mg deficiency increases Pb retention,
both in the mother and in the fetus [22]. Also, all the available
studies [except for two: ref. 40, 82] show an antagonism between
Mg and Pb. For example, Pb and Cd cross animal [1, 31, 89] and
human [19, 50, 64] placental barriers, and Mg is a competitive
inhibitor of Pb and Cd in the isolated human amnion [9, 43,
44].
Practical Conclusions on the Processing of Drinking
Waters
Drinking water containing Mg is, qualitatively and
quantitatively, useful, and therefore drinking and cooking water
should not be softened. In the case of Mg-poor water, the problem
of the best processing technique can be discussed. It is tempting
to have it enriched in order to get 30 mg/l Mg.
Simpson [103] suggests that one should increase the
Mg level of tap water after it has been collected from the tap.
Marier et al. [71] and Anderson et al. [5] do
not agree with the idea of adding Mg to the water in processing
stations. This increase in the Mg level, in processing stations,
might impair the balance due to super saturation of
CO3Ca and increase corrosivity.
Two methods have been proposed to reduce the corrosivity:
Richards and Moore [91] increase the pH and precipitate
the metals by adjunction of lime, but this method is inadequate
because the decrease of Pb is insufficient; besides, Na
orthophosphate which is used as a corrosivity inhibitor increases
the already excessive intake of P in our diet.
Neutralization by filtration on calibrated grains of
earth-alkaline metals reduces the Pb level in water. Marble has
long been used, but the reaction is too low. Neutralite
(CO3Ca + CO3Mg) or Magno or Akdolit
(CO3Ca + MgO) [15, 30] have also been used. With the
best anticorrosive power the best filter should induce the
highest Mg/Ca ratio.
Taux de magnésium de l’eau de boisson et facteur
de risque cardiovasculaire
La dureté de l’eau ne peut représenter la
caractéristique correspondant fidèélement
avec la donnée épidémiologique
définissant un <<facteur eau>> de risque
cardiovasculaire. La notion selon laquelle la mortalité
cardiovasculaire est en relation inverse avec la dureté de
l’eau ne se retrouve que dans 2 enquêtes sur 3. Par
contre, la correlation inverse se retrouve toujours
lorsqu’on remplace l’étude de la dureté
(somme de taux de Ca et Mg) par celle du taux isolé de Mg.
La ration alimentaire des pays développés
étant très souvent marginale, l’apport du Mg
de l’eau de boisson constitue le facteur critique laissant
la ration déficiente si l’eau est pauvre en Mg et la
faisant passer à un taux non carencé
lorsqu’elle est riche en Mg. Or la carence
magnésique expérimentale et clinique favorise la
pathologie cardiovasculaire. L’importance de l’apport
du Mg de l’eau de boisson ne se reduit pas à cette
donnée quantitative directe. Elle est aussi qualitative
car le Mg de l’eau semble être absorbé mieux
et plus rapidement que celui des aliments. Cette
particularité pourrait peut-être permettre de
comprendre que — même avec un apport
magnésique suffisant — une eau riche en Mg soit plus
saine: des données épidémiologiques et
expérimentales vérifient en effet que méme
en l’absence de carence magnésique, la prise
d’une eau riche en Mg prévient l’hypertension
artérielle, les troubles sodopotassiques, phosphocalciques
et nerveux observés tant chez l’homme que chez
l’animal d’experience. Le taux de Mg de l’eau
intervient aussi indirectement sur 1es apports en Mg par ses
effets au cours de la cuisson des aliments: une eau douce
appauvrit les aliments en Mg tandis qu’une eau dure les
enrichit. Le taux de Mg de la ration intervient enfin pour
antagoniser les polluants cardiovasculo-nocifs (par exemple, dans
l’amnios humain, Mg est un inhibiteur compétitif de
Pb et Cd). Aux effets propres du Mg sur l’appareil
cardiovasculaire, il convient donc d’adjoindre ses
propriétés antagonistes des métaux
cardiovasculotoxiques dont les eaux corrosives favorisent
l’apport. Comme il ne paraît pas possible
d’enrichir artificiellement en Mg les eaux de boisson sans
en accroître <<l’indice de cor
rosivité>>, cette pratique apparemment
séduisante, ne semble donc pas recommandable dans les
stations de traitement des eaux. Il convient d’y
neutraliser les eaux corrosives par filtration sur des grains
calibrés d’alcalino-terreux (Neutralite, Magno ou
Akdolit), en s’efforçant d’obtenir a pouvoir
anti-corrosif égal le rapport Mg/Ca hydrique le plus
élevé.
References
1 Ahokas, R.A.; Dilts, P.V., Jr.: Cd uptake by the rat embryo
as a function of gestational age. Am. J. Obstet. Gynec.
135: 2 19-222 (1979).
1a Altura, B.M.: Sudden death ischemic heart disease and
dietary magnesium intake: is the target site coronary vascular
smooth muscle? Med. Hypotheses 5: 843-849 (1979).
2 Altura, B.M.; Altura, B.T.: Role of Mg ions in contractility
of blood vessels and skeletal muscles. Magnesium-Bull.
3: suppl. 1A, pp: 102-114 (1981).
3 Altura, B.M.; Altura, B.T.: Mg, Na and K interactions and
coronary heart diseases. Magnesium 1: 241-265
(1982).
3a Altura, B.M.; Altura, B.T.; Gebrewold, A.; Ising, H.;
Günther, T.: Mg deficiency and hypertension: correlation
between magnesium deficient diets and microcirculatory changes in
situ Science 223: 1315-1317 (1984).
4 Altura, B.T.; Altura, B.M.: The role of Mg in etiology of
strokes and cerebrovasospasm. Magnesium 1:277-291
(1982).
5 Anderson, T.W.; Neri, L.C.; Schreiber, G.; Talbot, F.D.F.;
Zdrejewski, A.: Ischemic heart disease, water hardness and
myocardial Mg. Can. med. Ass. J. 113: 199-202
(1975).
6 Anderson, T.W.: Water hardness, Mg and ischemic heart
disease: Nova Scotia med. Bull. April: 58-61 (1977).
7 Arden, T.V.: Soft water and heart disease.. Water Services
80: 250-252 (1976).
8 Arden, T.V.: Cardiovascular mortality an analysis of the
water effect (Aqua Europa Publ, Henley on Thames (1977).
9 Bara, M.; Guiet-Bara, A.: Magnesium effect on the
permeability of the amniotic membrane as a whole and of the
epithelial cells, Magnesium-Bull. 3: 145-150 (1981).
10 Belizan, J.M.; Vilar, J.; Pineda, O.; Gonzalez, A.E.;
Sainz, E.; Garrera, G.; Sibrian; R.: Reduction of blood pressure
with Ca supplementation in young adults. J. Am. med. Ass
249: 1161-1165 (1983)
11 Berthelot,A.; Esposito,J.: Effects of dietary.Mg on.the
development of hypertension in the spontaneously hypertensive
rat. J..Am.Coll. Nutr. 4: 343-353 (1983).
12 Berthelot, A.; Gairard, A.: PTH- and DOCA-induced
hypertension in the rat. Clin. Sci. 58: 365-371
(1980).
13 Binnerts W.T.; Lowik M.R.H.; Groot, E M.: On the importance
of Mg in drinking water, in trace element. Analyt. Chem. Med.
Biol. vol. 2, pp 87-93 (De Gruyter, Berlin 1983).
14 Blaustein, M.P.; Hamlyn, J.M.: Role of natriuertic factor
in essential hypertension: an hypothesis. Ann. intern. Med.
98: 785-792 (1983).
15 Bonnefoy, X.; Certain, R.; Kisfaludi, G.: Essais de
neutralisation des eaux agressives d'un massif greseux et
granitique. Techniques Sci. municip. 79: 131-136
(1984).
16 Bonnefoy, X.; Huel, G.; Gueguen, R.: Variation of the blood
lead level as a function of lead contamination of the subjects
drinking water. Water Res.(in press 1984).
17 Boudene, C.; Food contamination by metals; in Di Ferrante,
Trace metal exposure and health effects, pp. 163-183 (Pergamon
Press, Oxford 1979).
18 Boudene, C.; Arsac, F.: Les métaux présents
dans notre alimentation quotidienne. Gaz. méd.
88: 2103-2121 (1981).
19 Buchet, J.P.; Roels, H.; Hubermont, G.; Lauwerys, R.:
Placental transfer of lead, mercury, cadmium and carbon monoxide
in women. II. Influence of some epidemiological factors on the
frequency distributions of the biological indices in maternal
cord blood. Environ. Res. 15: 494-503 (1978).
20 Calabrese, E.J.; Tuthill, R.W.: Elevated blood pressure
level and community drinking water characteristics. J. environ.
Sci. Health A13: 781-802 (1978).
21 Calabrese, E.J., Tuthill, R.W.: The influence of elevated
levels of Na in drinking water on elementary and high school
students in Massachusssetts. Sci. tot. Environ. 18: 117-133
(1981).
22 Cerklewsli, F.L.: Influence of maternal magnesium
deficiency on tissue lead content of rats. J. Nutr. 113:
1443-1447 (1983).
23 Chipperfield, B.: Chipperfield, J.R.: Heart muscle Mg, K
and Zn concentrations after sudden death from heart disease.
Lancet ii: 293-296 (1973).
24 Chipperfield, B.; Chipperfield, J.R.: Behr, G.; Burton, P.:
Mg in heart muscle. Lancet i: 1354-1355 (1976).
25 Chipperfield, B.; Chipperfield, J.R.: Cot deaths and
mineral salts. Lancet ii: 220 (1979).
26 Comstock, G.W.: Water hardness and cardiovascular diseases.
Am. J. Epidem. 110: 375-400 (1979).
27 Cottet, J.; Cristol, R.; Hydrotimétrie (ou
dureté de l'eau) et maladies cardiovasculaires. Bull.
Acad. natn. Méd. 166: 421-431. (1982).
28 Dauncey, M.J.; Widdowson, E.M.: Urinary excretion of Ca,
Mg, Na and K in hard and soft water areas. Lancet i:
711-714 (1972).
29 De Fulvio, S; Olori, L.: Definitions and classification of
naturally soft and naturally hard waters. Proc. Symp.
Hardness.of.Drinking Water and Public Health, Luxembourg, pp
95-122 (Pergamon Press, Oxford 1975).
30 Degremont, G.: Mémento technique de l’eau
(Technique et Documentation, Paris, 1978).
31 Dilts, P.V., Jr.; Ahokas, R.A.: Effects of dietary Pb and
Zn on fetal organ growth. Am. J. Obstet. Gynec. 136:
889-896 (1980).
32 Duc, M.; Abensur, R.: L’inquiétante
persistance du saturnisme hydrique. Concours méd.
104: 6659-6672 (1982).
33 Duc, M.; Abensur, R.; Barbier, P.; Chaput, C.: Les aspects
actuels du saturnisme hydrique. J. Toxicol. Med. 3:
323-339 (1983).
34 Durlach, J.: Les contrôles neurohormonaux du
métabolisme du Mg et leurs conséquences cliniques.
Revue fr. Endocr. clin. 21: 507-524 (1980).
35 Durlach, J.; Durlach, V.: Speculations on hormonal controls
of Mg homeostasis: a hypothesis. Magnesium 3:109-131
(1984).
36 Durlach, J.; Rayssiguier, Y.; Laguitton, A.: Le besoin en
Mg et son apport dans la ration. Méd. Nutr. 16:
15-21 (1980).
37 Dyckner, T.; Wester, P.O.: Effect of Mg on blood pressure.
Br. med. J. 286: 1847-1849 (1983).
38 Fine, B.P.; Barth, A.; Sheffet, A.; Lavenhar, M.A.:
Influence of magnesium on the intestinal absorption of lead.
Environ. Res. 12: 224-227 (1976).
39 Folsom, A.R.; Prineas, R.J.: Drinking water composition and
blood pressure: a review of the epidemiology. Am. J. Epidem.
115: 818-832 (1982).
40 Galt, R.M.; MacCreary, P.A.; Laing, G.H.; Hass, G.M.: Lead
vs. magnesium in dietary induction of rat tumors; in Hemphill,
Trace substances in environmental health, 189-194 (1980).
41 Guang-Sheng, Z.; Shang-Da, C.: An epidemiological study on
the relationship of urinary Na and K excretion to blood pressure
in China. Magnesium 1: 139-143 (1982).
42 Gueux, E.; Poenaru, S.; Duchene-Marullaz, P.:
Electromyographic changes associated with high level of magnesium
in drinking water. Magnesium-Bull. 4: 154-156
(1982).
43 Guiet-Bara, A.; Bara, M.: Transfert des cations monovalents
à travers la membrane amniotique humaine isolée.
C.r. Séanc. Soc. Biol. 173: 875-880 (1979).
44 Guiet-Bara, A.; Bara, M Durlach, J.: Magnesium: a
competitive inhibitor of lead. Study on the isolated human
amnion; in Halpern, Durlach, Magnesium deficiency. 1st Eur.
Congr. Magnesium, Lisbon 1983, pp. 38-45 (Karger Basel 1985).
45 Hackett,. P.L.; Kelman, B.J.: Availability of toxic trace
metals to the conceptus. Sci. total Environ. 28: 433-442
(1983).
46 Hankin, J.H.; Margen, S.; Goldsmith, N.F.: Contribution of
hard water to calcium and magnesium intakes of adults. J. Am.
diet. Ass. 56: 212-224 (1970).
47 Haring, B.S.A.; van Delft, W.: Changes in the mineral
composition of food as a result of cooking in ‘hard’
and ‘soft’ water. Archs envir. Hlth 36:
33-35 (1981).
48 Hasegawa, T.: Phosphorylation oxydative de mitochondries de
foie et de rein du rat après intoxication par le Cd et
effet préventif du Mg. Okoyama Igakai. Zasshi 91:
1447-1454 (1979).
49 Huel, G.; Derrienic, F.; Ducimetière, P.; Lazar,
P.Dureté de l’eau et mortalité
cardiovasculaire. Analyse critique des arguments de pathologie
géograpique. Revue- Epidém. Santé publ.
26: 349-359 (1978).
50 Huel, G.; Ibrahim, M.A.; Boudéne,. C.: Cd and Pb
content of maternal and newborn hair relationship to parity,
birth weight and hypertension. Archs. envir. Hlth 36:
221-227 (1981).
51 Huel, G.; Thomazeau, R.; Derriennic, F.; Lazar, P.:
Dureté de l’eau et mortalité
cardiovasculaire. Analyse portant sur 947 communes alsaciennes.
Revue Epidém. Santé publ. 26: 381-390
(1978).
52 Itokawa, Y.; Abe, T.; Tanaka, S.: Bone changes in
experimental chronic Cd poisoning. Archs Envir. Hlth 25:
241-244 (1973).
53 Itokawa, Y.; Abe, T.; Tanaka, S.: Renal and skeletal
lesions in experimental Cd poisoning in rats. Histology and renal
function. Environ. Res. 15: 206-217 (1978).
54 Kalkman, C.: Mg, hart vaatziekten en de waterfactor; thesis
No. 7430, Amsterdam (Huisdrukkerij, Faculteit der Geneeskunde,
Amsterdam 1979).
55 Karppanen, H.: Epidemiological studies on the relationship
between Mg intake and cardiovascular disease. Artery 9:
190-199 (1981).
56 Kemmer, F.N.: The NALCO water book (McGraw-Hill, Maidenhead
1979).
57 Klevay, L.M.: Mg, Ca, Cu and Zn in meals: correlations
related to the epidemiology of ischemic heart disease. Biol.
Trace Elements Res. 4:95-104 (1982).
58 Kobayashi, J.A.: Geographical relationship between the
chemical nature of river water and death rate from apoplexy; Ber.
Ohara Inst. Lansw. Biol. 11: 12-21 (1957).
59 Kopp, S.V.; Perry, H.M.; Perry, E.F.; Erlanger, M.: Cardiac
physiologic and tissue metabolic changes following chronic low
level Cd and Cd plus lead ingestion in the rat. Toxicol appl.
Pharmacol. 69: 149-160 (1983).
60 Krall, A.R.; MacLean, W.S.; Gamble, H.F.; Rogers, E.W.;
Reigart, J.R.; Collawn, S.S.: Effects of magnesium infusions on
the metabolism of calcium and lead; in Cantin, Seelig, Magnesium
in health and disease, pp. 941-948 (1980).
61 Krizek, V.; Sadilek, L.; Stepanek, P.: Die Trinkkur bei
Patienten mit Normo- und Hyperkalziurie. II.- Eine Studie
über die Marienbader Karolinenquelle. Balneol. Bohem.
11: 91-104 (1982).
62 Labeeuw, M.; Gerbaulet, C.; Pozet N.; Zech, P.; Traeger,
J.; Dissociation of Mg and Ca urinary excretions following water
ingestion. Magnesium 2: 156-163 (1983).
63 Lange1ier,W.F.:The analytical control of anti-corrosion
water treatment. J. Am. Water Works Ass. 28: 1500-1521
(1936).
64 Lauwerys R.; Buchet, J.P.; Roels H; Hubermont, G.:
Placental transfer of lead, mercury, cadmium and carbon monoxide
in women. I. Comparison of the frequency distributions of the
biological indices in maternal and umbilical cord blood. Environ.
Res. 15: 278-289 (1978).
65 Levin A.I.; Novikov, Y.V.; Plitman, S.I.; Noarov, .Y.A.;
Lastochkina, K.O.: Effect of water of varying degrees of hardness
on the cardiovascular system. Gig. Sanit. 10: 16-19
(1981).
66 Lowik, M.R.H.; Groot, E.M.; Binnerts, W.T.: Mg and public
health: the impact of drinking water; in Hemphill, Trace
substances in environmental health vol. 16, pp. 189-195
(1982).
67 Luoma, H.; Kivilvoto, L,; Punsar, S.; Knekt, P.; Risk of
myocardial infarction in Finnish men in relation to fluoride
magnesium and calcium concentration in drinking water. Acta med.
scand. 213: 171-176 (1983).
68 MacCarron, D.A.: Ca, Mg and P balance in human and
experimental hypertension. J Am. med. Ass. 4: suppl,
III, pp. III27-III33 (1982).
69 Mantel, O.; Marcel, G.A.; Lagrue, G.: Hypertension
artérielle, potassium et vaisseaux. Médecine act.
10: 219-229 (1983).
70 Manthey, J.; Stoeppler, L.; Morgenstern, W.; Nussel, E.;
Opherk, D.; Weintraut, A.; Wesch, H.; Kubler, W.: Magnesium and
trace metals: risk factor for coronary heart disease? Circulation
64: 722-729 (1981).
71 Marier, J.R.; Neri, L.C.; Anderson, .T.W.: Dureté de
l’eau, santé et importance du magnesium. NRCC/CNRC
No. 17582 (Ottawa 1980).
72 Marier, J.R.: Role du Mg dans la cardio-protection des eaux
dures. Méd. Nutr. 16: 23-29 (1980).
73 Marier, J.R.: Nutritional and myocardial aspects of Mg in
drinking water. Magnesium-Bull. 3: suppl. 1A, pp. 48-54
(1981).
74 Marier, J.R.: Quantitative factors regarding Mg status in
the modern-day world. Magnesium 1: 3-15 (1982).
75 Marier, J.R.: Role of environmental Mg in cardiovascular
diseases. Magnesium 1: 266-276 (1982).
76 Marier, J.R.: Le Mg provenant de l’eau potable. Gaz.
méd. Fr. 90: 1993 (1983).
77 Masironi, R.: Valeur des oligoéléments de
l’eau pour la santé. Chron. OMS 32: 413-417
(1978).
78 Masironi, R.; Shaper, A.G.: Epidemiological studies of
health effects of water from different sources. A. Rev. Nutr.
1: 375-380 (1981).
79 Maugeri, S.; Santi, C.: Calcio e magnesio nella pro filas
del saturnismo sperimentale. Medna Lav. 24: 1-14
(1938).
80 Merz, W.; Trace minerals and atherosclerosis. Fed. Proc.
41: 2807-2812 (1982)
81 Meyer, P.: Hypertension artérielle humaine
génétique environnement (Na). Med. Nutr.
19: 284 (1983).
82 Mylroie, A.A.; Hickmon, T.; Hill, G.: Effects of
supplemental magnesium on blood and tissue lead levels and
toxicity of ingested lead in the rat; in Hemphill, Trace
substances in environmental health, pp. 178-183 (l981).
83 Ninard, B.: Analyse des eaux minérales
françaises les plus riches en magnésium (au dessus
de 75 mg/l). Méd. Nutr. 13: 53 (1977).
84 Novikov, Y.V.; Plitman, S.I.; Levin, A.I.; Noarov, Y.:
Fixation de normes hygiéniques pour le taux minimal du Mg
dans l'eau potable. Gig. Sanit. No. 9, pp. 7-11 (1983).
85 Pitkanen, H.: Industrial possibilities to interfere with
the salt problem: dietary Na/ (K+Mg) ratio. Magnesium 1:
298-303 (1982).
86. Pocock, S.J.; Shaper, A.G.; Cook, D.G.; Packham, R.F.;
Lacey, R.F.; Powell, P.; Russel, P.F.: British Regional Heart
Study: geographic variations in cardiovascular mortality and the
role of water quality. Br. med. J. 280: 1243-1249
(1980).
87 Poirier, L.A.; Kasprzak, K.S.; Hoover, K.L.; Wenk, M.L.:
Effects of Ca and Mg acetates on the carcinogenicity of Cd
chloride in Wistar rats. Cancer Res. 43: 4575-4582
(1983).
88 Punsar, S.; Karvonen, M.J.: Drinking water quality and
sudden death: observations from West and East Finland. Cardiology
64: 24-34 (1979).
89 Renaud, S.; Ciavatti, M.; Thevenon, C.; Ripoll, J.P.:
Protective effects of dietary Ca and Mg on platelet function and
atherosclerosis in rabbits fed saturated fat. Atherosclerosis
47: 187-198 (1983).
90 Revis, N.W.; Major, T.C.; Horton, C.Y.: The effects of
calcium, magnesium, lead or cadmium on lipoprotein metabolism and
atherosclerosis in the pigeon. J. environ. Path. Toxic.
4: 293-304 (1980).
91 Richards, W.N.; Moore, M.R.: Plumbosolvency in Scotland:
the problem, remedial action taken and health benefit observed.
Proc. Conf. Am. Water Works Ass., Miami, pp. 901-918 (1982).
92 Ryznar, J.W.: A new index for determining amount of calcium
carbonate scale formed by a water. J. Am. Water Works Ass.
36: 472-473 (1944).
93 Sasaki, N.: Na and K intake of the Japanese. Magnesium
1: 151-155 (1981).
94 Schleiffer, R.; Berthelot, A.; Pernot, F.; Gairard, A.:
Parathyroids, thyroid and development of hypertension in SHR.
Jap. Circul. J. 45: 1272- 1279 (1981).
95 Schlettwein-Gsell, D.; Mommsen-Straub, S.: Übersicht
Spurenelemente in Lebensmitteln: VII: Mg. Z.
Vitam.-Horm.-Fermentforsch. 42: 324-352 (1972).
96 Schroeder, H.A.: Degenerative cardiovascular disease in the
Orient. II. Hypertension. J. chron. Dis. 8:312-333
(1958).
97 Schroeder, H.A.: Relation between mortality from
cardiovascular disease and treated water supplies. Variations in
states and 163 largest municipalities in US. J. Am. med. Ass.
172: 1902-1908 (1960).
98 Schroeder, H.A.: Municipal drinking waters and
cardiovascular death rates. J. Am. med. Ass. 195:
125-129 (1966).
99 Schroeder, H.A.; Kraemer, L.A.: Cardiovascular mortality,
municipal water and corrosion. Archs envir. Hlth
28:302-311 (1974).
100 Seelig, M.S.: Mg in the pathogenesis of disease. Early
roots cardiovascular skeletal and renal abnormalities, p. 488
(Plenum Press, New York 1980).
101 Seelig, M.S.: Mg requirements in human nutrition.
Magnesium-Bull. 3:suppl. lA, 26-47(1981).
102 Sharrett, A.R.: The role of chemical constituents of
drinking water in diseases. Am. J. Epidemiol: 110: 401-418
(1979).
103 Simpson, D.J.: Mg supplementation in drinking water. Vet.
Rec. 71: 174 (1971).
104 Singh, N.P.; Thind, I.S.; Vitale, L.F.; Pawlow, M.: Intake
of magnesium and toxicity of lead: an experimental model. Archs
envir; Hlth 34: 168-173 (1979).
105 Skrabal, J.; Hörtnagl, H.; Auböck, J.A.: The
role of the sodium pump in the control of vascular tone on the
rat. Lancet I: 463-470 (1980).
106 Speich, M.; Bousquet, B.; Nicolas, G.: Intéret
physio pathologique de l'étude des rapports Mg/Ca et K/Na
dans les ventricules cardiaques de sujets témoins et de
malades décédes après un infarctus du
myocarde. Revue fr. Endocr. clin. 20: 545-549
(1979).
107 Speich, M.; Metayer, C.; Arnaud, P.; Nguyen, V.G.; Boitea,
H.C.: Concentrations of Pb, Mg, Ca, Zn and Cd in 20 rabbit
tissues after exposure to low lead doses and atherogenic diet.
Ann. Nutr. Metab. 27: 531-541 (1983).
108 Thomas,J.; Gaultier. J.; Thomas, E.; Desgrez, P.: Sur la
teneur en Mg des eaux de boisson. Revue fr. Endocr. clin.
19: 151-153 (1978).
108a Turlapaty, P.D.M.V.; Altura, B.M.: Magnesium deficiency
produces spasms of coronary arteries: relationship to etiology of
sudden death ischemic heart disease. Science 208:
198-200 (1980).
109 Van Barneveld, A.A.: Trace element absorption and
retention in mice. The role of experimental conditions and the
influence of Ca and Mg, p. 215 (Fotodruck J. Mainz, Aachen
1983).
110 Van Barneveld, A.A.; Van den Hamer, C.S.A.; Houtman,
J.P.V.: Drinking water hardness, trace elements and
cardiovascular diseases: influence of Ca and Mg on metabolism of
Cd, Ca, Cu, Zn and Se in mice;in Hemphill Trace substances in
environmental health, vol. 16, pp. 196-204 (1982).
111 Wanger, P.D.; Weswig, P.H.; Stoner, J.C.: Arsenic levels
in Oregon waters. Environ. Hlth Perspect. 19: 139-143
(1977).
112 Wardener, H.E. de; MacGregor, G.A.: The role of a
circulating inhibitor of Na+-K+ATPase in essential hypertension.
Am. J. Nephrol. 3: 88-91(1983).
113 Wesenberg, G.B.M.: Effect of Cd. on Ca, Mg and P content
of rat molars. Scand. J. dent. Res. 90:
95-101(1982).
114 Wong, C.S.; Berrang, P.: Contamination of tap water by
lead pipe and solder. Bull. env. Contam. Toxicol. 15:
530-534 (1976).
115 Zaversnik, H.; Ozim, V; Ozim, V.: Magnesium in our
drinking water. Zdrav. Vestn. 52: 179-182 (1983).
Received: July 16, 1984
Accepted: October 2, 1984
J. Durlach,
CHU Cochin-Port-Royal,
27, Rue du Faubourg Saint-Jacques,
F-75674 Paris Cédex 14 (France)
This page was first uploaded to The Magnesium Web Site on
September 23, 2002
http://www.mgwater.com/
