Journal of Nutritional Medicine (1991) 2, 165-178
REVIEW
The Importance of Magnesium in the Management of Primary
Postmenopausal Osteoporosis
GUY E. ABRAHAM MD FACN
Optimox Corporation, 2720 Monterey Street, Suite 406,
Torrance, CA 90503, USA
Data are presented which support the theory that most
cases of primary postmenopausal osteoporosis (PPMO) are not
caused by calcium deficiency. The commonly applied therapy of
continuous supplementation solely with large doses of calcium
is unlikely, therefore, to be of help. It is furthermore
suggested that magnesium deficiency has a significant role in
PPMO: magnesium is involved in calcium metabolism and in the
synthesis of vitamin D, and in maintaining bone integrity. The
results of a clinical evaluation of a dietary programme
involving magnesium supplementation are also
presented.
Keywords: magnesium, osteoporosis, calcium,
postmenopause, primary postmenopausal osteoporosis.
INTRODUCTION
During the last National Institute of Health (NIH) Workshop on
Osteoporosis, the panel of experts recommended that 1500 mg of
calcium should be ingested daily by postmenopausal women to
prevent bone loss from primary postmenopausal osteoporosis (PPMO)
[1], reiterating advice by others since 1981 [2-5]. The bone loss
occurring during the first decade following menopause is
predominantly at the expense of the trabecular bone with 50% loss
whereas only 5% of the cortical bone is lost during the same time
interval [6]. Evidence has been presented that calcium
supplements of 660-3000 mg per day had no significant effect on
trabecular bone loss in postmenopausal women [7, 8], and caused
hypercalcemia and hypercalciuria in 24% of women receiving
1000-3000 mg per day [9]. Presented here are data supporting the
concept that PPMO in most cases is not caused by calcium
deficiency, and that it is not preventable by calcium megadosing.
Furthermore, data will be presented suggesting that magnesium
deficiency plays an important role in PPMO and adequate magnesium
intake and reserve may be the most efficient, safe and
cost-effective approach to the prevention and management of
PPMO.
CONSIDERATION OF THE RELATIVE IMPORTANCE OF CALCIUM AND
MAGNESIUM IN PRIMARY POSTMENOPAUSAL OSTEOPOROSIS
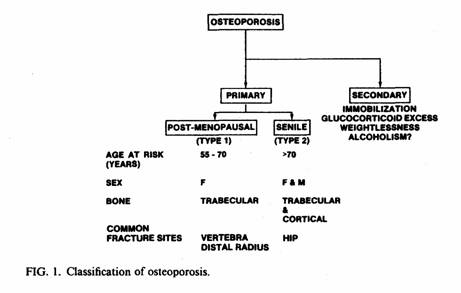
Osteoporosis
The bone loss, without change in bone structure, of
osteoporosis leads to high susceptibility to bone fracture [10,
11]. When it is caused by excess glucocorticoids [12],
immobilization [13] or weightlessness [14, 15], it is termed
secondary osteoporosis. When it develops in both sexes over 70
years of age, it is termed primary senile osteoporosis, and is
characterized by loss of both cortical and trabecular bone. In
postmenopausal women, it is termed primary postmenopausal
osteoporosis (PPMO), which is characterized by radiologically
manifest loss, predominantly of trabecular bone, occurring during
the first decade after menopause [16, 17] (Fig. 1).
Dietary Calcium and Magnesium Intakes, Possibly
Related to Spontaneous PPMO
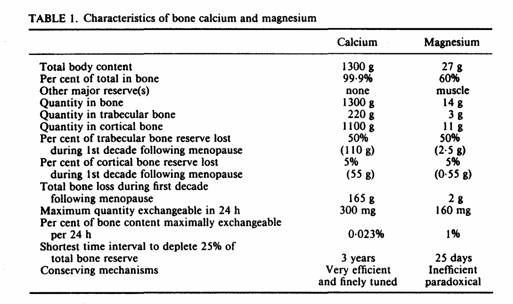
Although bones contain 99% of the total body calcium, (Table
1) there is a poor correlation between bone density and calcium
intake [18-22]. The lowest hip fracture rates in postmenopausal
women are found in populations with the lowest calcium intakes
(400-500 mg per day) [23]. In premenopausal Caucasian women,
lifetime calcium intakes averaging 500-800 mg per day was
associated with optimal mass of both cortical and trabecular
bone, that was not greater in those with calcium intakes above
800 mg per day [24]. During the first five years following
menopause, calcium supplementation has no effect on either
trabecular or cortical bone even when calcium intake from food
was as low as 400 mg per day [25]. After five years
postmenopause, calcium supplementation using 500 mg of the
citrate salt has a positive effect on trabecular bone when intake
of calcium from food was below 400 mg. This effect was not
present when calcium carbonate was used.
Vegetarian postmenopausal women, who consume less calcium, but
twice as much magnesium as omnivorous women [26, 27], have
greater mean density of cortical bone [27, 29]. The difference is
significant after the fifth decade of life [27], usually the
first decade after menopause. When the bone mineral densities
(BMD) of the hip, spine and forearm were correlated with the
intake of 14 nutrients in 159 Caucasian women [30], no
significant correlation was found between calcium intake and bone
mass at any site. Zinc correlated positively to forearm BMD in
premenopausal women only whereas, iron and magnesium were
significant predictors of forearm BMD in pre menopausal and
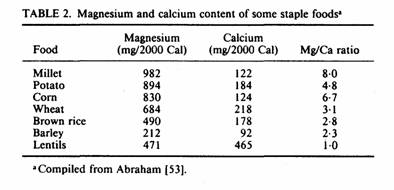
postmenopausal women. In many rural areas, cereals and potatoes
provide more than 70% of the energy consumed [31]. These staple
foods contain much more magnesium than calcium (Table 2), and can
provide as much as 1000 mg per day of magnesium with consumption
of 2000 kcal from these sources (almost four times more magnesium
than the most recent recommended dietary allowance (RDA) for
magnesium for women—280 mg per day [32]. Such a diet would
provide less than 50% of the RDA for calcium.
In laboratory animals, experimental calcium deficiency induces
osteomalacia [22], whereas magnesium deficiency induces
osteoporosis [33].
Physiological Factors, Involving Magnesium, that
Affect Calcium Metabolism and Bone Density
Magnesium, inadequacy of which is common in the occidental
diet [26, 34, 35, 36], plays important roles in calcium
metabolism, through its requirements for normal activity of the
hormones controlling calcium utilization and for maintenance of
normal bone structure.
Adequate magnesium intake and reserve is required for the
synthesis of calcitriol, the active dihydroxy metabolite of
vitamin D [37]. Magnesium deficiency causes abnormal calcium
utilization, extending to hypocalcemia, by impairing parathyroid
hormone (PTH) secretion, release and interfering with end-organ
response to PTH [37-40]. This effect of magnesium on PTH
secretion and action could be explained by the requirement of
magnesium for the activity of adenylate cyclase in parathyroid
tissue [41], kidney [42], and in bone [43].
Balance studies suggest that man can adapt to relatively low
calcium intake by increasing its absorption and decreasing its
renal excretion [44]. Decreasing the calcium intake from 1000 mg
to 500 mg per day resulted in a negative calcium balance during
the first months, but after eight months, the balance became
positive [45]. The loss of calcium during this time was 8-10 g,
which is less than 1% of total bone. The hormonal response to
this adaptation mechanism was present one week after switching
from 2000 to 300 mg per day calcium in nine normal women [46]. No
efficient mechanism has been found for rapid adaptation to low
magnesium intake [47].
Adaptation to low calcium intake entails synthesis of the
hormone, 1,25-(OH)2D3 (calcitriol) [48], by ingestion of foods
containing vitamin D3, or synthesizing it from a cholesterol
derivative in the skin, by its 25-hydroxylation in the liver, and
by its 1-alpha-hydroxylation to 1,25-(OH)2D3 (calcitriol) in the
kidneys [48-50]. Decreased calcitriol levels have been reported
in PPMO [51] and small doses of calcitriol normalize calcium
absorption in PPMO [52]. The enzyme involved in the renal
hydroxylation step, that activates vitamin D, is
magnesium-dependent [37], and is inhibited by intramitochondrial
accumulation of calcium and phosphate [48], which is
magnesium-dependent [53]. Clinical evidence that magnesium
deficiency, which is common in women with PPMO [54, 55],
contributes to poor responsiveness to vitamin D has long been
recognized with the therapeutic effect of vitamin D on intestinal
calcium absorption and hypocalcemia being not fully achieved in
the absence of adequate magnesium [56, 57].
Sodium intake induces urinary calcium losses [58, 59]. In
young subjects, there is a compensatory mechanism which is
magnesium-dependent that increases absorption of calcium by
PTH-induced 1,25-(OH)2D3 synthesis [58]. This adaptation
mechanism is impaired in postmenopausal women [59], probably due
to magnesium deficiency.
Since potassium enhances 1-hydroxylation of 25-(OH)D3 [60], it
is conceivable that potassium depletion could impair synthesis of
1,25-(OH)2D3 and predispose to PPMO. In cases of magnesium
deficiency, the cells cannot retain potassium because of a
defective potassium pump. In such cases, potassium
supplementation will be ineffective unless magnesium deficiency
is also corrected.
PTH and calcitonin (CT), the second and third hormones that
play important roles in calcium metabolism and bone density [61],
are also influenced by magnesium so as to inhibit calcium removal
from bone, and deter its deposition in soft tissues. The major
skeletal effect of PTH is to increase bone resorption by
stimulating osteoclasts, thereby mobilizing bone calcium. It also
favors soft tissue calcium uptake and phosphate renal excretion.
CT conversely increases calcium deposition in bone matrix and
blocks soft tissue calcium uptake.
Increased serum magnesium and serum ionized calcium stimulate
CT and suppress PTH secretion. Hyperparathyroidism increases in
frequency at and after the menopause [62, 63], and PPMO is more
severe in hyperparathyroid than in hypoparathyroid women [64]. In
most women with PPMO, however, PTH is the same or lower than in
normal postmenopausal women 65-67]; CT is not lower [68]. Since
serum magnesium is normal [69] or low [55], with evidence of low
bone magnesium [54] in women with PPMO, but albumin-adjusted
serum calcium is elevated [68], the pattern of PTH and CT in PPMO
may reflect the elevated serum calcium resulting from bone
mineral mobilization.
In premenopausal women, estrogens suppress the PTH-mediated
mobilization of bone minerals. The protective effect of estrogen
on bone may be explained by its inhibiting effects both on PTH
release [70] and on PTH-demineralizing effect on bone [71, 72].
Serum and urine magnesium levels are higher in postmenopausal
women than in premenopausal women; estrogen administration in
postmenopausal women abolishes the change in serum and urine
magnesium after the menopause [73, 74] probably by blocking
mobilization of bone magnesium. Therefore another possible
mechanism of estrogen action on bone is maintenance of bone
magnesium reserve.
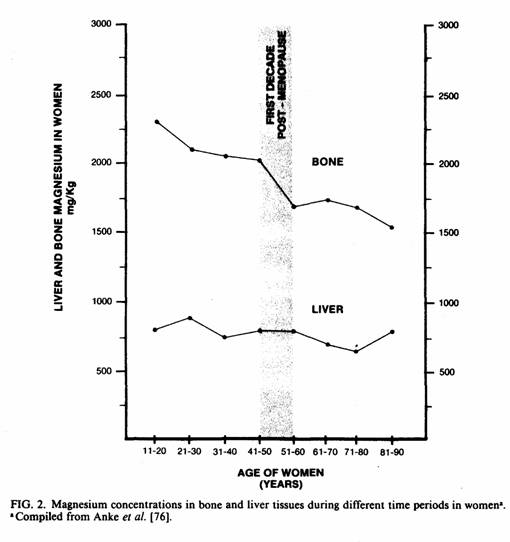
Although only 17% of total bone mass is trabecular bone [75],
more than twice as much trabecular as cortical bone loss occurs
during the decade after menopause; as much as half the trabecular
bone is lost, versus 5% of cortical bone [6]. During that time,
there is a sharp decrease in bone magnesium [76] whereas liver
magnesium remains constant (Fig. 2). As indicated by serum and
erythrocyte magnesium levels, it seems that in women with PPMO,
mobilization of trabecular bone magnesium is insufficient to
maintain blood and soft tissue magnesium levels [54, 55, 69]. In
contrast to the depressed trabecular magnesium, bone calcium
levels are normal [54].
SUGGESTED DAILY INTAKE OF CALCIUM, MAGNESIUM AND VITAMIN D
FOR THE POSTMENOPAUSE
Calcium
Because of its availability and low cost, calcium carbonate
from oyster shell is the most commonly promoted calcium
supplement [77]. However, it has a propensity for formation of
uroliths [78] and interferes with iron absorption [and it has
relatively poor bioavailability [79]. The citrate salt of calcium
is less likely to cause urolithiasis [75], is more bioavailable
[80], and because of its acidity is less likely to interfere with
absorption of iron.
A recent prospective study suggests that moderately increased
calcium intake may lower the incidence of hip fracture of senile
osteoporotic patients [81]. However, when patients with severe
PPMO were given massive doses of calcium, they developed positive
calcium balance, but without radiographic evidence of improvement
in the osteoporotic process [82, 83, 84]. Since experimental
excess calcium has long been associated with soft tissue
calcinosis, especially in the presence of magnesium deficiency
[71], and high dosage calcium treatment of patients with
osteopathies has interfered with magnesium retention [85, 86],
megadosing PPMO patients with calcium may present a risk of
abnormal calcium deposition [53]. As much as 10% of calcium in
the elderly is extraskeletal [87].
Excess calcium may also predispose to luteal deficiency in
premenopausal women, 1 mM calcium chloride having been found to
decrease luteal hormone (LH) binding to the plasma membrane of
the corpus luteum and causing luteolysis [88]. It also increases
synthesis of prostaglandin F2x, which is luteolytic [88, 89].
There is no evidence that calcium supplementation in excess of
500 mg prevents or reverses PPMO [7, 8, 25]. For the above
reasons, 500 mg of calcium in the form of citrate is
recommended.
Vitamin D
High doses of vitamin D have caused soft tissue calcification
in experimental animals, and have been implicated in renal and
other comparable lesions in humans [71]. An extensive review of
vitamin D intake in the USA has disclosed that the average
American may unwittingly consume several thousand units of
vitamin D from fortified foods [90]. This overdosage with vitamin
D can increase the risk of soft tissue calcification from excess
calcium. Since the depressed calcitriol levels of patients with
PPMO have not been related to vitamin D deficiency, but to its
decreased synthesis, contributed to by magnesium deficiency,
there is no justification to administer more than the recommended
daily dose of 400 IU of vitamin D to postmenopausal women.
Magnesium
Dalderup, in The Netherlands [91], was the first to suggest
that magnesium supplementation might be beneficial in the
management of osteoporosis, and warned against the risk of soft
tissue calcification from excess calcium and vitamin D treatment.
This is a particular problem for the American postmenopausal
woman, whose vitamin D intake is likely to be high, who is urged
to consume more calcium, and whose magnesium intake is likely to
be low. Surveys have shown that 39% of American women between 15
and 50 years of age receive less than 70% of the RDA for
magnesium (at 300 mg per day) [35, 36]. A review of the
literature indicates that the magnesium content of the food
supply in North America and Europe provides about 72-161 mg less
than the 300 mg magnesium RDA [26]. The most recent US RDA is 280
mg per day. In the USSR, the magnesium RDA for women is 500-1250
mg, depending on physiological factors [92]. Since the US RDA is
largely based on short-term balance studies, the most recent of
which are in stress-free metabolic ward conditions (which
decrease magnesium needs), the US RDA may reflect the minimum
daily requirement, without allowance for increased needs of
anabolism, nutrient and hormonal imbalances, and stress [93].
Low magnesium intake may increase vulnerability to PPMO [53].
The only three therapies of PPMO that show a significant and
positive effect on trabecular bone are fluoride, 1,25-(OH)2D3
[94] and estrogens [7]. Fluoride increases the incorporation of
magnesium in bone and the proper F+/Mg2+ ratio is important for
bone integrity [95]. Side-effects and poor response to fluoride
therapy may be due to magnesium deficiency. A recent study
suggests that supplementation of the diet with 400-600 mg of
magnesium daily reduced significantly (p < 0.0l) the
side-effects of fluoride therapy in postmenopausal women with
PPMO [96]. As previously discussed, synthesis of 1,25- (OH)2D3 is
impaired in magnesium deficiency [37]. Estrogen increases bone
uptake and retention of magnesium [71].
This author recommends supplementation of magnesium to reach a
total daily intake of 1000 mg, and has supplemented the diet with
up to 600 mg per day of magnesium as the oxide without
gastrointestinal side effects or loose stools [97].
The above recommendations for magnesium should be part of a
total dietary program since several nutrients besides magnesium
and calcium are important for bone integrity and some of those
nutrients have been reported to be lower in serum and bone of
women with PPMO than normal controls [98]. Since food items high
in magnesium are also high in these nutrients found to be
important for bone integrity [99], a magnesium-emphasized dietary
program would also increase the intake of micronutrients which
are important for the well being and bone integrity of the
postmenopausal woman.
CLINICAL EVALUATION OF THE MAGNESIUM-EMPHASIZED DIETARY
PROGRAM
The magnesium-emphasized program was implemented for six to 12
months in 19 postmenopausal women receiving hormonal replacement
therapy. Seven postmenopausal women on hormonal replacement
therapy served as controls. Trabecular bone density was assessed
with single photon densitometry of the calcaneous bone with a 3%
error [100].
The vertebral body contains 70%-80% trabecular bone [101] and
the calcaneous bone is 95% trabecular [102]. Bone mineral density
(BMD) of the spine correlates very well with calcaneous BMD
[100]. For measuring rate of bone loss at a single site
calcaneous BMD compares well to other techniques with regard to
the relationship between reproducibility and the anticipated rate
of change [103]. Ross et al. [100] found that calcaneous BMD
correlates with fracture risk and calculated the fracture
threshold at which the fracture risk doubles relative to
premenopausal women. This fracture threshold was 0·32 g
cm2.
Twenty-six postmenopausal women were recruited from a
menopause clinic. All subjects were on hormonal replacement
therapy, either estrogen alone in those with surgical menopause
or cyclic progestogen superimposed on estrogen therapy in those
with an intact uterus. All patients underwent BMD of the
calcaneous bone with single photon absorptiometry, as described
by Ross et al. [100], and were advised about the dietary program
(Table 3). Micronutrients were supplied in the form of a complete
multivitamin, multimineral supplement (Gynovite® Plus,
Optimox Inc., Torrance, CA) containing 500 mg of calcium as the
citrate salt and 600 mg of magnesium as the oxide (Table 4).
Seven patients received dietary advice but chose not to take the
supplement. Nineteen patients received dietary advice and
ingested six tablets of the nutritional supplement daily.
Therefore, the 19 patients received 50% of the recommended daily
allowance (RDA) of calcium and 200% of the RDA of magnesium for
women. In all 26 patients, BMD of the calcaneous bone was
repeated at their return visit, 6-12 months later.
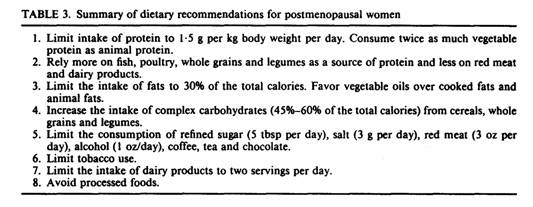

Comparing the two groups of patients, there was no significant
difference in age, height, weight, years since menopause,
duration of hormone therapy, baseline BMD or duration of
follow-up (Table 5).
A non-significant increase of 0·7% in the mean BMD of
the seven patients receiving hormonal therapy and dietary advice
was observed as compared with a mean increase of 11% in the 19
women receiving the supplements (p < 0.01 by paired data
analysis).
The effect of this magnesium-emphasized program on calcaneous
bone density was 16 times greater than that of dietary advice
alone in postmenopausal women on hormonal replacement therapy.
Ross et al. [100] defines the spine fracture threshold as a BMD
of 0·32 g cm-2 of the calcaneous bone. In 15 of the 19
women receiving the supplement the BMD was below the fracture
threshold before treatment. Within a year after the program only
seven patients had BMD values less than 0·32 g cm-2.
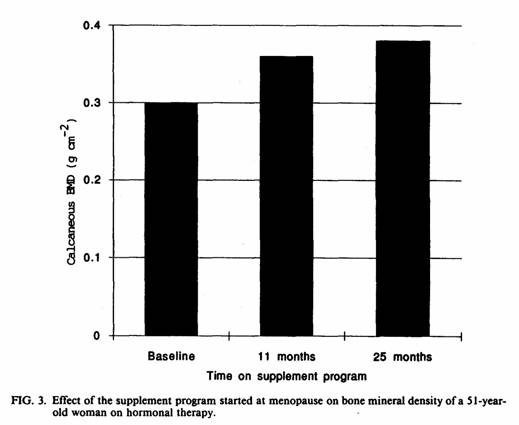
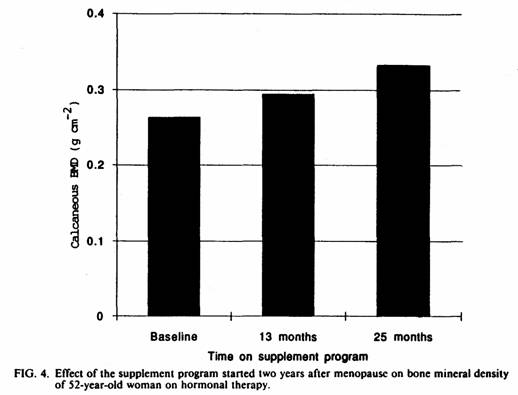
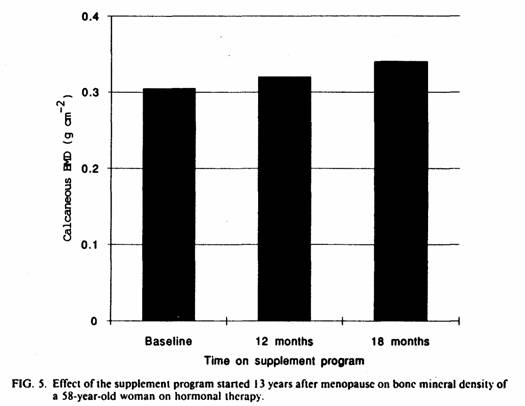
The positive effect of this magnesium-emphasized
supplementation on the BMD was still present at two year
follow-up (Fig. 3-5). Best results were obtained when this
program was implemented soon after menopause. The results of this
study suggest that the effect of the magnesium-emphasized total
dietary program on calcaneous BMD is not short-term and temporary
but long-term and persistent.
REFERENCES
[1] Chestnut CH. Report from the NIH consensus conference,
1984, and NIH/NOF workshop, 1987. In: Genant HK, ed. Osteoporosis
update, 1987. San Francisco, CA: Radiology Research and Education
Foundation, 1987, 3-6.
[2] Draper HH, Seythes CA. Calcium, phosphorus, and
osteoporosis. Fed Proc 1981; 40: 2434.
[3] Aviolo LV. Postmenopausal osteoporosis: prevention versus
cure. Fed Proc 1981; 40: 2418.
[4] Seeman E, Riggs BL. Dietary prevention of bone loss in the
elderly. Geriatrics 1981; 36: 71-9.
[5] Editorial. Risk factors in post-menopausal osteoporosis.
Lancet 1985; i: 1370-1.
[6] Nordin BEC. Clinical significance and pathogenesis of
osteoporosis. Br Med J 1971; 1: 571.
[7] Ettinger B. Estrogen, progestogen, and calcium in
treatment of postmenopausal women. In: Genant HK, ed.
Osteoporosis update, 1987. San Francisco, CA: Radiology Research
and Education Foundation, 1987, 253-58.
[8] Christiansen C, Riis, BJ. Optimal prophylaxis for
postmenopausal bone loss. In: Genant HK, ed Osteoporosis update,
1987. San Francisco, CA: Radiology Research and Education
Foundation, 1987, 259-66.
[9] Riggs BL, Seeman E, Hodgson SF, Taves DR. O’Fallon
WM. Effect of fluoride regimen on vertebral fracture occurrence
in postmenopausal osteoporosis. N Eng J Med 1982; 306: 446.
[10] Consensus Development Conference. Osteoporosis. .JAMA
1984; 252: 799-802.
[11] NIH Consensus Development Conference. Osteoporosis. JAMA
1984; 252.
[12] Baylink DJ. Glucocorticoid-induced osteoporosis. N Eng J
Med 1983; 309: 306.
[13] Stewart AF, Adler M, Byers CM. Calcium homeostasis in
immobilization: an example of rescriptive hypercalciuria. N Eng J
Med 1982; 306: 1136.
[14] Lutwak L, Whedon GD, Lachance PA. Mineral, electrolyte
and nitrogen balance studies of the Gemini-VII fourteen-day
orbital space flight. J Clin Endocrinol Metab 1969; 29:
1140-56.
[15] Whendon GD, Lutwak L, Rambuat PC. Mineral and nitrogen
metabolic studies, experiment MO71. In: Johnston RS, Dietlein IF,
eds Biomedical Results from Skylab. Washington. DC: Scientific
and Technical Information Office. National Aeronautics and Space
Administration, 1977, 164-90.
[16] Riggs BL, Mellon LJ III. Heterogeneity of involutional
osteoporosis: evidence for two osteoporosis syndromes. Am J Med
1983; 75: 899.
[17] Johnston CC, Norton J, Khairi MRA. Heterogeneity of
fracture syndromes in postmenopausal women. J Clin Endocrin Metab
1985; 61: 551.
[18] Nordin BEC, international pattern of osteoporosis. Clin
Orthop Rec Res 1966; 45: 17-30.
[19] Hurxthal LM, Vose GP. The relationship of dietary calcium
intake to radiographic bone density in normal and osteoporotic
persons. Calc Tiss Res 1969; 4: 245-56.
[20] Smith RW, Frame B. Concurrent axial and appendicular
osteoporosis. New Eng J Med 1965; 273: 73-78.
[21] Garn SM, Solomon MA, Friedl J. Calcium intake and bone
quality in the elderly. Ecology Food Nutr 1981; 10: 131-33.
[22] Kanis JA, Passmore R. Calcium supplementation of the
diet—I ∓ II. Br Med J 1989; 298: 137-40, 205-8.
[23] Hegsted DM. Calcium and Osteoporosis. J Nutr 1986; 116:
2316-19.
[24] Halioua L, Anderson J.JB. Lifetime calcium intake and
physical activity habits: independent and combined effects on the
radial bone of healthy premenopausal Caucasian women. Am J Clin
Nutr 1989; 49: 534-41.
[25] Hughes RD, Dallal GE, Krall EA, Sadowski L, Sahyoun N,
Tannebaum S. A controlled trial of the effect of calcium
supplementation on bone density in postmenopausal women. New Eng
J Med 1990; 323: 878-83.
[26] Marier JR. Magnesium content of the food supply in the
modern-day world. Magnesium 1986; 5:1-8.
[27] Marsh AG, Sanchez TV, Mickelsen O. Cortical bone density
of adult lacto-ovo-vegetarian and omnivorous women. J Am Diet
Assoc 1980; 76: 148-51.
[28] Ellis FR, Holesh S, Ellis JW. Incidence of osteoporosis
in vegetarians and omnivores. Am J Clin Nutr 1972; 25: 555.
[29] Sanchez TV, Micklesen O, Marsh AG, Garn SM. Bone mineral
in elderly vegetarian and omnivorous females. In: Mazess, RB, ed.
Proceedings of the Fourth International Conference on Bone
Measurement. Bethesda: NIH, 1980; 94.
[30] Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary
intake and bone mineral density. Bone and Mineral 1988; 4:
265-77.
[31] Davidson S, Passmore R, Brock JF, Truswell AS. Human
Nutrition and Dietetics. Edinburgh: Churchill Livingstone, 1979;
166-75.
[32] Recommended Dietary Allowances. Washington, DC: National
Academy Press, 1989.
[33] Mirra J, Alcock NW, Shils ME. Effects of calcium and
magnesium deficiencies on rat skeletal development and
parathyroid gland area. Magnesium 1982; 1: 16-33.
[34] Seelig MS. The requirement of magnesium by the normal
adult. Am J Clin Nutr 1964; 14: 342-90.
[35] Morgan KJ, Stampley GL, Zabik ME. Magnesium and calcium
dietary intakes of the U.S. population. J Am Coll Nutr 1985; 4:
195-206.
[36] Pao EM, Mickle SJ. Problem nutrients in the United
States. Food Tech 1981; 35: 60-69.
[37] Rude RK, Adams JS, Ryzen E. Low serum concentrations of
1,25-Dihydroxyvitamin D in human magnesium deficiency. J Clin
Endocrinol Metab 1985; 61: 933.
[38] Rude RK, Oldham SB, Sharp CF, Singer FR. Parathyroid
hormone secretion in magnesium deficiency. J Clin Endocrinol
Metab 1978, 47: 800-6.
[39] Rude RK, Oldham SB, Singer FR. Functional
hypoparathyroidism and parathyroid hormone end-organ resistance
in human magnesium deficiency. Clin Endocrinol 1976; 5:
209-24.
[40] Connor TB, Toskes P, Mahaffey J, Martin LG, Williams JB,
Walser M. Parathyroid function during chronic magnesium
deficiency. Johns Hopkins Med J 1972; 131: 100-17.
[41] Dufresne LR, Gitelman HJ. A possible role of adenyl
cyclase in the regulation of parathyroid activity by calcium. In:
Talmage RV, Manson PL, eds Calcium, Parathyroid Hormone and the
Calcitonins. Excerpta Medica. 1972; 197-201.
[42] Ghazarian JG. DeLuca HF.
25-Hydroxycholecalciferol-l-Hydrolase: A specific requirement for
NADPH and a hemoprotein component in chick kidney mitochondria.
Arch Biochem Biophys 1974; 160: 63.
[43] Rude RK. Skeletal adenylate cyclase: effect of Mg Ca and
PTH. Calcif Tiss Intl 1985; 37: 3 18-23.
[44] Davidson S, Passmore R, Brock JF, Truswell AS. Human
Nutrition and Dietetics. Edinburgh: Churchill Livingstone. 1979;
90-106.
[45] Malm LJ. Calcium requirement and adaptation in adult man.
Scand J Clin Lab Invest 1958; 10 (suppl): 36.
[46] Hughes BD, Stern DI, Shipp CC, Rasmussen HM. Effect of
lowering dietary calcium intake on fractional whole body calcium
retention. J Clin Endocrinol Metab 1988; 67: 62.
[47] Rude RK, Bethune JE, Singer FR. Renal tubular maximum for
magnesium in normal. hyperparathyroid and hypoparathyroid man. J
Clin Endocrinol Metab 1980; 51: 1425-31.
[48] Norman AW, Henry H. 1,25-Dihydroxycholccalciferol—A
hormonally active form of vitamin D3. Rec Progr Hormone Res 1974;
30: 431-80.
[49] Holick MF, Clark MB. The photobiogenesis and metabolism
of vitamin D. Fed Proc 1985; 44: 1149.
[50] MacIntyre I. Vitamin D and the integration of calcium
regulating hormones. In: Dumont J, Nunez J, eds. First European
Symposium on Hormones and Cell Regulation. Amsterdam:
North-Holland. 1977; 195-208.
[51] Gallagher JC, Riggs B, Eisman J. Intestinal calcium
absorption and serum vitamin D metabolites in normal subjects and
osteoporotic patients: effect of age and dietary calcium. J Clin
Invest 1979; 64: 729-36.
[52] Riggs LB, Nelson KI. Effect of long term treatment with
calcitriol on calcium absorption and mineral metabolism in
postmenopausal osteoporosis. J Clin Endocrin Metab 1985; 61:
457.
[53]Abraham GE. The calcium controversy. J .Appl Nutr 1982;
34: 69.
[54] Cohen L, Kitzies R. Infrared spectroscopy and magnesium
content of bone mineral in osteoporotic women. Israel J Med Sci
1981; 17: 1123.
[55] Reginster JY, Strause L, Deroisy R. Preliminary report of
decreased serum magnesium in postmenopausal osteoporosis.
Magnesium 1989; 8: 106-9.
[56] Medalle K, Waterhouse C, Hahn TJ. Vitamin D resistance in
magnesium deficiency Am J Clin Nutr 1976; 29: 858.
[57] Heaton FW, Fourman P. Magnesium deficiency and
hypocalcemia in intestinal malabsorption. Lancet 1965; ii:
50.
[58] Breslau NA, McGuire J, Zerwwekh, JuE. The role of dietary
sodium on renal excretion and intestinal absorption of calcium
and vitamin D metabolism. J Clin Endocrinol Metab 1982; 55:
369.
[59] Breslau NA, Sakhaee K. Impaired adaptation to salt
induced urinary calcium losses in postmenopausal osteoporosis.
Trans Assoc Am Physicians 1985; 98: 107.
[60] Bikle D, Murphy W, Rasmussen, H. The ionic control
1.25-dihydroxyvitamin Dc synthesis in isolated chick renal
mitochondria. The role of potassium. Biochem Biophys Acta 1976;
437: 394.
[61] Aurbach GD, Marx SJ, Spiegel AM. Parathyroid hormone,
calcitonin and calciferols. In: Williams, RH, ed. Textbook of
Endocrinology. London: Saunders, 1981; 922-1032.
[62] McGeown M. Sex, age and hyperparathyroidism. Lancet 1969;
i: 887-88.
[63] Muller H. Sex, age and hyperparathyroidism. Lancet 1969;
i: 449-50.
[64] Hossain M, Smith DA, Nordin BEC. Parathyroid activity and
menopausal osteoporosis, Lancet 1970; i: 809-11.
[65] Teitelbaum, SI, Rosenberg EM, Richardson, CA.
Histological studies of bone from normocalcemic postmenopausal
osteoporotic patients with increased circulating parathyroid
hormone. J Clin Endocrinol Metab 1976; 42: 537-43.
[66] Franchimont P, Heynen G. Parthorone and calcitonin in
osteoporosis. In: Parthorone and Calcitonin Radioimmunoassay in
Various Medical and Osteoarticular Disorders. Philadelphia, PA:
JB Lipincott Company, 1976; 101-7.
[67] Dequeker J, Bouillon R. Parathyroid hormone secretion and
25-hydroxyvitamin D levels in primary osteoporosis. Calcif Tissue
Res 1977; 22: 495-6.
[68] Prince RI, Dick IM, Prince RI. Plasma calcitonin levels
are not lower than normal in osteoporotic women. J Clin
Endocrinol Metab 1989; 68: 685.
[69] Reginster JY, Denoprdhoot BM, Albert A. Serum and
erythrocyte magnesium in osteoporotic and osteoarthritic
postmenopausal women. In: Proceedings of the Symposium on
Magnesium: Experimental and Clinical Results. Soc Mag Research,
1985: 17.
[70] Boucher A, D’Amour P, Hamel L. Estrogen replacement
decreases the set point of parathyroid hormone stimulation by
calcium in normal postmenopausal women. J Clin Endocrinol
Metab1989; 68: 831-36.
[71] Seelig MS. Magnesium deficiency in the pathogenesis of
disease. New York: Plenum Medical Book Company 1988; 119,
299-301, 317-21. 333-6.
[72] Seelig MS. Increased magnesium need with use of combined
estrogen and calcium for Osteoporosis. Magnesium Res 1990;
3:197-215.
[73] Lindsay R, Hart DM, Forrest C. Effect of a natural and
artificial menopause on serum, urinary and erythrocyte magnesium.
Clin Science 1980; 58: 255-57.
[74] McNair P, Christiansen C, Transbol LB. Effect of
menopause and estrogen substitutional therapy on magnesium
metabolism. Mineral Electrolyte Metab 1984; 10: 84-88.
[75] Gong JK, Arnold JS, Cohn SH. Composition of trabecular
and cortical bone. Amat Rec 1964; 149: 325-32.
[76] Anke M, Grun M, Schneider HJ. Der magnesiumstatus des
menschen in abhangigkeit von alter und geschlecht. Magnesium,
Stofwechsel, Colloquium, Universitat von Iena 1976; 36-51.
[77] Rogers LF. Financial consideration in osteoporosis. In:
Genate HK. ed. Osteoporosis Update1987. San Francisco, CA:
Radiology Research and Education Foundation Publishers. 1987;
41-5
[78] Harvey JA, Zobitz MM, Pak CYC. Calcium citrate: Reduced
propensity for the crystallization of calcium oxalate in urine
resulting from induced hypercalciuria of calcium supplementation.
J Clin Endocrinol Metab 1985; 61: 1223.
[79] Hughes-Dawson B, Seligson FH, Hughes VA. Effects of
calcium carbonate and hydroxyapatite on zinc and iron retention
in postmenopausal women Am J Clin Nutr 1986; 4: 83.
[80] Nicar MJ, Pak CYC. Calcium bioavailability for calcium
carbonate and calcium citrate. J Clin Endocrinol Metab 1985; 61:
9l.
[81] Holbrook TL Calcium in the diet and the risk of hip
fracture. Lancet 1988; 2: 1046-49.
[82] Riis B Thomsen K Christiansen C Does calcium
supplementation prevent postmenopausal bone loss? A double blind,
controlled clinical study. N Engl J Med 1987; 316: 173.
[83] Davidson S, Passmore R Brock JF Trusswell AS. Hum
Nutrition and Dietetics. Edinburgh: Churchill Livingstone. 1979;
90-106.
[84] Kanis JA, Passmore. R Calcium supplementation of the
diet-- I ∓ II. Br Med J 1989; 298: 137-40, 205-8.
[85] Amiot D, Hioco D, Durlach J. Frequence du deficit
magnesique chez le sujet et dans diverses osteopathies. J Med
Besancon 1969; 5: 37 1-78.
[86] Parlier R, Hioco D, LeBlanc R. Le metabolisme du
magnesium et ses rapports avec celui du calcium. I. A propos
d’une etude des bilans magnesiens chez l’homme
normal, dans les osteopathies et les nephropathies. Rev Franc
Endocr Clin 1963; 4: 93-135.
[87] Mazess RB. Calcium intake and bone. Am J Clin Nutr 1985;
42: 568-671.
[88] Dennefors, BL, Sjogren A, Hamberger L. Progesterone and
adenosine 3’,5’-monophosphate formation by isolated
human corpora lutea of different ages: influence of human
cholonic gonadotropin and prostaglandins. J Clin Endocrinol Metab
1982; 55: 102-7.
[89] Riley JC, Carlson JC. Involvement of phospholipase A
activity in the plasma membrane of the rat corpus luteum during
luteolysis, Endocrinology 1987; 121: 776-81.
[90] Scientific Literature Reviews on Generally Recognized as
Safe (GRAS) Food Ingredients: Vitamin D. Washington, DC: National
Tech, Information Service, US Dept. of Commerce.1974; 252.
[91] Dalderup, LM. The role of magnesium in osteoporosis and
idiopathic hypercalcaemia. Voeding 1960; 21: 424-34.
[92] Lederer J. Magnesium: Mythes et Realite. Paris: Maloine
Editeurs, 1984; 54.
[93] Seelig MS. Magnesium requirements in human nutrition.
Magnesium Bulletin 1981; 1a: 26.
[94] Osteoporosis Update 1987. In: Genant HK, ed. San
Francisco, CA: Radiology Research and Education Foundation
Publishers, 1987; 279, 297.
[95] Okazaki M. Magnesium action on the stability of
fluorapatite. Magnesium 1988; 7: 148-53.
[96] Muenzenberg KJ, Koch W. Mineralogic aspects in the
treatment of osteoporosis with magnesium. J Am Col Nutr 1989; 8:
461.
[97] Abraham G, Grewal H. A total dietary program emphasizing
magnesium instead of calcium. J Rep Med 1990; 35: 503.
[98] Gaby AR, Wright JV. Nutrients and osteoporosis. J Nutr
Med 1990; 1: 63-72.
[99] Watt BK, Merrill AL. Composition of foods. In:
Agriculture Handbook No. 8. Washington, DC: US Department of
Agriculture, October, 1975.
[100] Ross PD, Wasnich RD, Heilbrun LK, Vogel JM. Definition
of a spine fracture threshold based upon prospective fracture
risk. Bone 1987; 8: 27 1-78.
[101] Eastell R, Mosekilde L, Hodgson SF, Riggs LB. Proportion
of human vertebral body bone that is calcaneous. J Bone and
Mineral Res 1990; 5: 1237.
[102] Wasnich RD. Thiazides in osteoporosis. In: Genant HK,
ed. Osteoporosis Update 1987. San Francisco, CA: Radiology
Research and Education Foundation Publishers. 1987; 301.
[103] Lancaster EK, Evans RA, Kos S, Hills E, Dunstan CR, Wong
SYP. Measurement of bone in the os calcis: a clinical evaluation.
J Bone and Mineral Res 1989; 4: 507.
This page was first uploaded to The Magnesium Web Site on July
19, 2002
http://www.mgwater.com/










