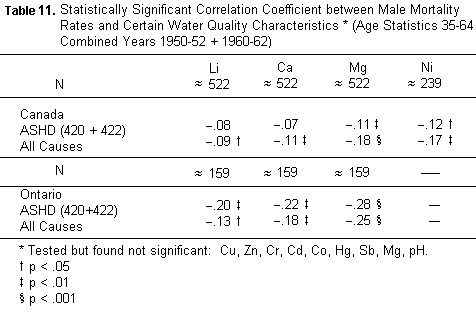Annals New York Academy of Sciences, 1978 , pp 203-219.
WATER HARDNESS AND CARDIOVASCULAR MORTALITY
Luciano C. Neri
Department of Epidemiology and Community Medicine
University of Ottawa
Ottawa, Ontario, Canada
Helen L. Johansen
Bureau of Epidemiology
Laboratory Center for Disease Control, Health and Welfare
Ottawa, Ontario, Canada
Ever since Kobayashi (1) in 1957, noted a parallel between the
geographic distribution of the acidity of water in Japanese
rivers and the distribution of what was then one of the major
causes of mortality in Japan, apoplexy, an increasing number of
investigators all over the world have attempted to elucidate and
confirm a geographic relationship between quality of drinking
water and mortality, particularly from cardiovascular causes. The
now voluminous literature in this field has been subject to
several comprehensive reviews (2-9).
As remarkable as the geographic diversity of these studies is
the great diversity of the hypotheses that have been favored by
different investigators, both as regards the identity of the
water-borne factor to which they impute a good or bad influence,
and as regards the nature of the disease or pathologic process
induced. Recently (5), we expressed regret at the lack of any
emerging consensus among those who have contributed to the
literature over the last 15 years, and at the failure of most
studies to yield evidence capable of discriminating among any of
64 major classes of explanatory hypotheses that need to be
considered. Kobayashi did not refer to any category other than
apoplexy, but ecological studies, from Schroeder on, have usually
tried several cause-specific death rates as dependent
variables.
Thus, even though most investigators continue to refer to the
hazard of residing in soft-water areas as if it related
specifically to cardiovascular disease, support for this view has
been progressively weakened by the admission of a bronchitis
effect (10) and by the suggestion that there is also an infant
mortality effect in England, Wales (11, 12) and possibly Canada,
(13) though not in the United States (14). Stocks (15) showed
that whatever may be the environmental or genetic factor
responsible for regional mortality patterns of cardiovascular
disease in the United Kingdom, it would appear to have a
pronounced effect also on the incidence of congenital
malformation of the central nervous system, and to a lesser
extent this would apply to nephritis and carcinoma of the
stomach. In the United States, Sauer (16) found as strong a
negative correlation of hardness with malignant neoplasm as with
cardiovascular deaths. Canadian statistics (17) show that more
than half the excess mortality in soft-water areas is certified
to noncardiovascular causes of death.
Uncertainty as to the real effect of the water factor has lead
some investigators to discriminate between various components of
cardiovascular mortality in the hope of identifying a more
specific association. For example, in Ontario, coronary deaths
were subdivided into coroner- and noncoroner-certified deaths
(18). It was reported that water effects related specifically to
the former, which were taken to represent sudden deaths. The
interpretation offered was that a mechanism of increased
susceptibility to lethal arrhythmias was operating in soft-water
areas. Although the use of coroner certification as a proxy for
suddenness of death has been challenged (19), the proposed
mechanism is still of interest, especially if it can be extended
into the domain of deaths certified to non-cardiovascular
disease. Likewise, the suggestion, originally made by Schroeder
(20), whereby the mode of action of soft water was through an
increased risk of hypertension, is promising to the extent that
it can be applied across the board, not merely within the small
fraction of deaths in which hypertension is certified as an
underlying or contributing cause.
Schroeder's theory of cadmium-induced hypertension as the
missing link in the water story was based on four
considerations:
1. Cadmium induction of hypertension in rats (21);
2. The finding of higher cadmium concentration in human subjects
who died of hypertension (22);
3. The theoretical availability of cadmium from pipes through
corrosive action of soft water (20);
(I use the word "theoretical" because Schroeder did not have as
we now have in Canada any widely-based survey data on cadmium in
relation to softness.)
4. The relationship between soft water and cardiovascular
mortality (21), and thence to hypertension.
This is no doubt a very attractive theory that has support
from laboratory, experimental and clinical evidence. However,
epidemiologic support has been slow in forthcoming. An
alternative hypothesis, still attributing increased mortality to
increased frequency of hypertension in soft-water areas, has been
proposed by Crawford. Initially, she postulated a protective
effect of calcium against lead absorption in soft-water areas
(25, 26), and later a more complex mechanism involving the ratio
of magnesium plus calcium to sodium (2). A similar theory was
proposed by Joossens (27), who nominated sodium as the noxious
element, with calcium exerting the protective action. Nitrates in
drinking water, hitherto considered related only to
methemoglobinemia, have also been implicated in an increased
frequency of hypertension and its complications.
I will discuss these four postulated water-borne hypertensive
agents in turn.
Morton (TABLE 1) reported that the Republican River counties
in east central Colorado, which have the hardest water, have the
highest level of nitrates, the highest hypertensive death rates
and the highest prevalence of hypertension among draft
registrants (28). He speculated that since organic nitrates have
been associated with increased risk of diastolic hypertension in
explosives workers (29-33), regions with more intensive
agriculture and greater use of nitrogen fertilizers may
experience an increase in hypertension risk. However, Sauer
pointed out (34) that cardiovascular death rates are generally
low in Colorado, and that if one sums the four cardiovascular
causes of death offered by Morton, the mortality on the
Republican River counties becomes the lowest.
TABLE 1
NO3 2- CONCENTRATION VERSUS AGE-ADJUSTED MORTALITY RATES
(1959-61) AND PREVALENCE OF HYPERTENSION IN SELECTIVE SERVICE
REGISTRANTS (1957-64)*
-----------------------------------------------------------------
Mortality/100,000
Four
Hyper- Cardiovas- Prevalence/
River Basin N032- ** tension cular Causes 1000
----------------------------------------------------------------
Republican R. 13.7 57 340 16.0
Platte R. 3.0 24 371 8.5
Arkansas R. 1.9 26 358 8.4
Rio Grande R. 1.0 31 343 4.4
San Juan R. 0.6 18 357 7.8
Colorado R. 0.4 25 365 5.8
-----------------------------------------------------------------
* Data from: W. E. Morton, 1971. (28)
** Population weighted mean values in ppm for countries grouped
by river basin.
The strongest epidemiologic support implicating the mechanism
of hypertension in the water story comes from Stitt, in the
United Kingdom. He reported (35) on a sample of 244 civil
servants living in six hard-water localities and as many living
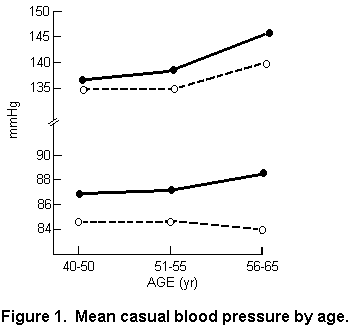
in six localities with soft water. FIGURE 1 summarizes the
results of these observations. It can be seen that not only are
both systolic and diastolic blood pressures higher in the
soft-water areas but that, particularly for diastolic, and quite
noticeably for systolic blood pressure, there is a divergence of
mean pressure with increasing age. The localities under study
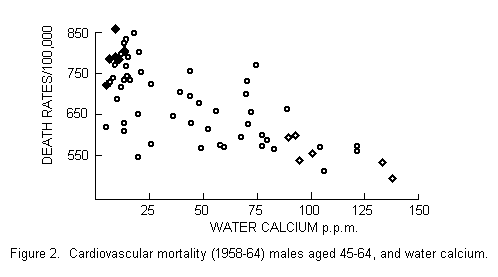
were chosen to represent a contrast between high mortality in
areas having soft water and low mortality in areas having hard
water (FIGURE 2). This leaves us with some uncertainty as to the
generality of an association between water quality and blood
pressure. Larger cohorts presently being studied in the United
Kingdom may enable the investigators to confirm their findings.
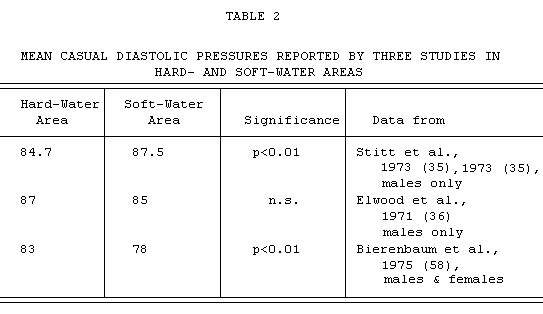
It seems, however, to be in conflict with the observations of
Elwood in Wales (36), where similar-sized random samples from the
population of hard- and soft-water areas were also examined, and
no significant differences in mean blood pressures were found
(see TABLE 2).
Stitt did not propose a specific mechanism to explain his
findings; however, the British group was then advocating the
theory of a protective effect from calcium against plumbosolvency
in soft waters (37). The origin of this theory comes from a large
autopsy study conducted by Crawford and Crawford (38) in Glasgow
and in London. They found that in Glasgow (very soft water) there
was a significant excess of lead in the bones of both accidental
and myocardial deaths (26). Additionally, they found:
1. Accidental deaths in Glasgow had higher prevalence of healed
infarcts, more atheroma and lumen stenosis;
2. Ischemic heart disease sudden deaths in Glasgow had less
extensive atheroma and lumen stenosis than in London.
They suggested that the findings in both cities indicated an
increased susceptibility of the myocardium in soft-water
areas.
Further support for the possible involvement of lead comes
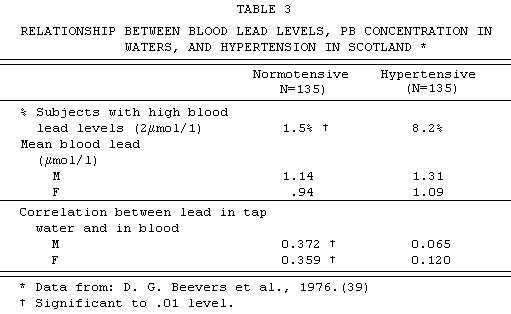
from Beevers et al (39). They examined the blood and
tap-water lead levels of 135 pairs of age-sex-matcbed
hypertensive and normotensive subjects. TABLE 3 shows their
findings: significant excess of persons with high blood lead
levels among hypertensives; and, among normotensives, a
significant positive correlation between blood lead and tap-water
lead concentrations.
A protective effect of calcium has also been related to the
water story by Langford and Watson (40) who proposed that a low
calcium intake may accentuate the hypertensive effect of sodium.
The relationship between sodium and high blood pressure has been
confirmed by clinical and experimental studies for the past 30
years. Clinical observations made as early as 1944 by Kemper (41)
indicate that a low sodium diet was helping hypertensive
patients. Experimentally, Meneely (42) demonstrated that rats
could be made hypertensive by feeding them large amounts of
sodium chloride. Joossens (27) in 1973 showed that there are
populations in which blood pressure does not increase with age
and that these are primitive populations that use no salt in
their diets. Shaper (43) observed rapid rises in blood pressure
in young Samburis, used to eating without salt, when they were
given 15 grams of salt a day during military service. A converse
phenomenon was reported by Sakaki (44) in Japan who noted that by
reducing salt intake in children taking school meals, their blood
pressure declined progressively.
Epidemiologic confirmation of the possible influence of salt
in drinking waters come from Steinbach et al (45). When
the village of Juilovka was found to have one of the highest
prevalence levels of hypertension in the world [45%], they
searched for an environmental factor and found that the water
contained a concentration of sodium 26 times greater than in
Bucharest and in the nearby village in the Gurghiu Valley. Fodor
(46, 47) observed that the rate of hypertension among
Newfoundland fishing villagers was much higher than in mainland
Canada and that the consumption of salt, particularly
salt-preserved food, was also very high. He was unable to
demonstrate a difference in salt consumption between hyper-and
normotensive subjects, but he drew attention to the fact that
essential elements such as magnesium, calcium, potassium, and
zinc were very much below minimum requirements; the possibility
of a protective effect from anions was again raised. The
increased tendency in industrialized countries to soften water
supplies, mostly in order to prevent scaling or poor detergent
action, illustrates the possible public health importance of
sodium. Schroeder (48) indicated the extent to which this
municipal softening bid progressed when he found that 2.5 times
as many urban municipalities (16.5%) softened their water in 1952
as compared to 1932. In defense of sodium, it has been pointed
out that water softening only increases the amount of sodium ions
that will be available in the form of carbonate or bicarbonate
(49) rather than chloride, which is the chemical form implicated
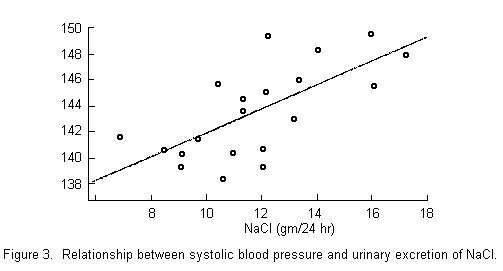
in hypertension. Certainly evidence such as that offered by
Joossens in FIGURE 3 is highly suggestive of an effect of sodium
chloride. In Canada, we have attempted to study the effect of
domestic water softeners. Two very large case-control studies
have been completed and will be reported shortly. These studies
involve a total of about 20,000 households, and they are both
prospective and retrospective in design.
For none of the above-mentioned elements, however, is the
evidence of a hypertensive effect from water as cogent as it is
in the case of cadmium. This evidence has been recently reviewed
by Perry, (50-52) and particular interest attached to his
observation that the hypertensive effect of cadmium in rats was
inhibited when hard water was used as the vehicle for
administration of the metal (53). Since publication of the
initial hypothesis by Schroeder and collaborators, Support has
been forthcoming: [1] at the experimental level, where
Schroeder's findings have been replicated and extended to other
metals (54); [2] at the clinical (autopsy level) where evidence
of increased cadmium or cadmium to zinc ratio in kidneys of
hypertensives has been confirmed in most studies (50, 54, 55)
(some of which tie low zinc concentrations more to renal damage
than to essential hypertension) (56), and [3] in part, at least,
at the ecological level, studying the association between cadmium
in drinking waters and prevalence of hypertension (57).
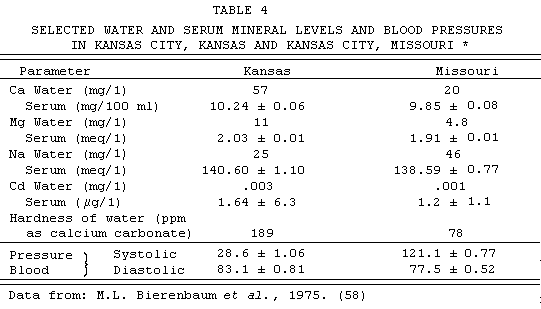
Bierenbaum (58) studied an apparent reversal of the usual
water story in Kansas City, where higher mortality from
cardiovascular disease was noted in the Kansas (hard water) part
as compared to the Missouri (soft water) part. Both Kansas and
Missouri components of the city derive their municipal water
supply from the same source. However, in Missouri the water is
softened to less than one-half the original value. TABLE 4
summarizes the findings, which have been held to suggest that the
differences in systolic and diastolic pressure found in their
case-control series might be explained by differences in cadmium
concentration, both in the drinking water and in the sera of
individuals involved. These data, however, have certain puzzling
features, some of which have been commented on, by Sharrett and
Feinleib (59).
Inconsistent with the cadmium theory is the fact that cadmium
workers have never been shown to suffer excess hypertension
(60-62).
We have not been able to obtain data on prevalence of
hypertension in smaller geographic units of the United States
than the three used by the National Health Survey (63, 64).
FIGURE 4 shows how these relate to the map of state average water
hardness prepared by Muss (65) on the basis of weighted municipal
values. The findings are far from clear: one could expect, on the
basis of hardness, that the lowest prevalence ofhypertension
would occur in the west and the highest in the northeast. TABLE 5
indicates that south has the lowest and northeast the highest
mean blood pressures, yet these regions are indistinguishable in
terms of their weigted average hardness. The next column shows
the actual prevalence of definite hypertension and its deviation
from an expectation based on age and sex composition of the white
population of the areas involved. Here the west has the largest
deficit as predicted. However, as great a difference is found
between south and northeast, and this is not fully explainable in
terms of water hardness.
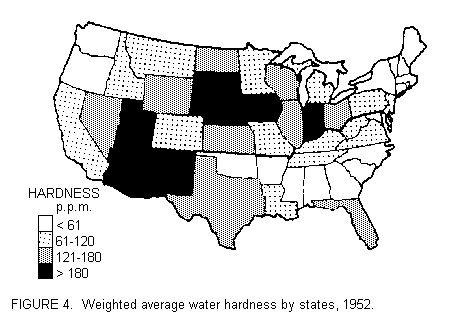
TABLE 5
WATER HARDNESS, PREVALENCE OF HYPERTENSION, AND MEAN SYSTOLIC
BLOOD PRESSURE FOR WHITE ADULTS, U.S. NATIONAL HEALTH SURVEY,
1960-62
-----------------------------------------------------------------
Rate of Definite
Hypertension
Difference
Weighted Mean Mean Systolic from
Region Hardness* Blood Pressure Actual Expected
---------------------------------------------------------------
North East 78 132.6 16.8 +2.60
South 76 127.6 12.8 -.04
West 147 130.5 12.2 -2.40
----------------------------------------------------------------
* Calculated from data of H.A.Schroeder, 1960. (48)
What does all this add up to?
From an epidemiologic point of view we are mainly interested
to see whether any of the appealing mechanisms proposed can be
considered the explanation of the water story, rather than
speculating whether the agents proposed are capable of exerting a
hypertensive effect at the clinical level. In this context, I
would like to propose to you some criteria for judging their
plausibility.
1. The first essential requisite for an agent to be considered
compatible with the water story as we know it, is that its
concentration in water supplies must follow the geographic
distribution of water hardness.
2. Its postulated effect, whether in terms of prevalence or
mortality, has to be consistent with the geographic variation in
mortality, including mortality attributed to cardiovascular
disease.
3. Its concentration in drinking waters must be sufficient to
exert the postulated effect.
It follows from the third criterion that the intake from water
should be critical in relation to the adequacy or deficiency of
intake from other sources. In judging this adequacy or
deficiency, it must be borne in mind that the chemical state in
which some elements may be found in water, could make it more or
less important than the simple arithmetic of food intake and
water intake suggests. It is not clear that any of the substances
so far discussed meet all these criteria. Nitrates have not been
implicated in epidemiologic data from anywhere outside of
Colorado. Against lead militates the fact that the reports
implicating its association have been limited to England and that
its cardiotoxicity and/or hypertensive effect, at usual levels of
intake, have not been well demonstrated either in animals or in
humans (66-67). Its concentration has been found to vary little
between hard and soft waters (68), but its supposed interrelation
with calcium may make this unimportant (69-71). Except perhaps in
the Bulgarian case, sodium intake from water, even from softened
waters, is too little as compared to other sources (72). In
Canada, the higher sodium concentrations are found in the prairie
provinces where mortality is lowest, and in fact, sodium
correlates negatively with mortality (73). The same observation
has been made by Schroeder (74) and by Sauer (75) in the United
States where the substance having the strongest correlation with
mortality was indeed sodium, but the correlations were
negative.
Cadmium is detectable in only a small percentage of municipal
waters (14% of those sampled in Canada), and its concentration in
these source waters does not correlate with hardness. The same is
true in the United Kingdom (76). At the point of use, cadmium may
as Schroeder speculated, and as Canadian data weakly confirm, be
more abundant in very soft water (8). Even so, cadmium intake
from drinking waters does not seem substantial, particularly if
we consider the amount that can be absorbed in other ways, such
as smoking (71).
Hence, if cadmium is to be cast for a role in the water story,
it will probably have to be in terms of postulated interaction
with some other element that is more abundant in hard than in
soft waters. (It is not clear whether zinc meets this
description.) Epidemiologically the effects on health determined
by a magnesium:cadmium or a calcium:cadmium ratio would be
indistinguishable from a situation in which concentrations of the
bulk elements alone determined the geographic variation in risk.
Certainly hardness as such, or calcium or magnesium alone, can be
shown, in various bodies of data, to be related in a striking way
to cardiovascular disease in general and to hypertension in
particular.
For example Masironi reported a study comparing the mortality
of residents in four United States river basins, two with hard
and two with soft water, found the death rates from hypertensive
heart disease to be 17% to 70% (see TABLE 6) higher in the
soft-water basin (78). Also from Masironi, we have a report
relating the mean systolic pressure in New Guinea villages along
the Wogupmeri River to calcium concentration (see FIGURE 5). As
calcium in the river water (which is drunk directly by the
villagers) declines from 8 to 3 ppm, the mean pressure rises from
about 97 to 110 mmHg (79).
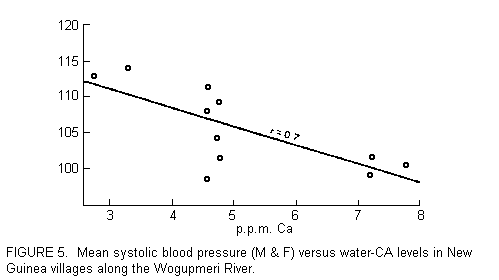
TABLE 6
DEATH RATES FROM HYPERTENSIVE HEART DISEASE(HHD)IN FOUR RIVER
BASINS, TWO WITH SOFT WATER AND TWO WITH HARD WATER *
-----------------------------------------------------------------
Water Hardness Death Rates
River Basin (per 100,000)
-----------------------------------------------------------------
Ohio River soft 39.7
Columbia River soft 33.3
Missouri River hard 28.3
Colorado River hard 23.3
----------------------------------------------------------------
* Data from: R. Masironi, 1970.(78)
The relationship between water hardness and mortality in
Canada is summarized in TABLE 7 in terms of the decrement of
death rate corresponding to an increment of hardness from 0 to
350 ppm (roughly the difference between some parts of
Newfoundland or British Columbia and Saskatchewan/Alberta). As
has already been stressed, more than half of this mortality
"effect" is expressed in noncardiovascular categories
(-91.9/-157.5 = 58%).
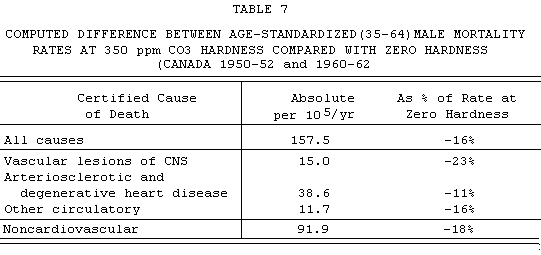
For the interest of this audience, I will draw attention to
the fact that the largest proportionate decrease (-23%)
occurs in the category with the closest affinity to hypertension.
However, rather than pursuing the hypothesis of a hypertensive
effect as the underlying mechanism of the water factor, in Canada
we are systematically examining the idea of an agent, present in
hard-water areas, protective against premature death, perhaps
especially against sudden deaths. Magnesium appears to be a more
likely agent of such a protective effect than calcium or any of
the 15 elements whose concentration we have surveyed in waters
from the kitchen tap of 526 Canadian localities (68). The
hypothesis is also in accord with the known behavior of
magnesium, as reviewed in recent publication (80-83). Of interest
in this context is the experimental evidence indicating the
adequacy of supplementary magnesium as a protection against
cardiotoxic substances (84) and against stress such as cold (85).
This effect has been confirmed by rat experiments in Russia (86)
where the yield, the extent and the rate of involution of cardiac
necrosis have been measured. Support may also be found in human
studies (87-89).
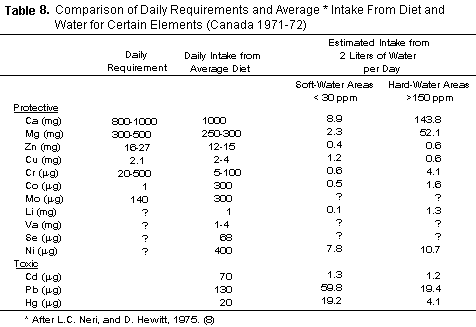
TABLE 8 indicates that magnesium intake from hard waters may
be substantial. The average North American diet is of doubtful
adequacy in this respect (90-92) as exemplified in the findings
of Brown et al. concerning the Boston brothers of men,
who remained in Ireland (93). Thus the 50 mg or more which may be
received from water by residents in areas where water is hard may
become critical especially in circumstances in which such
requirements are raised by a stressful situation, such as an
episode of arrhythmia.
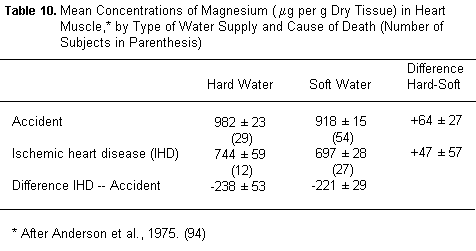
Supporting evidence also comes from our recently reported
autopsy study (94) in which concentration of elements in the
myocardium (and in the diaphragm and pectoralis as control
muscles), was studied in residents of hard and soft water areas
in Ontario who died either of myocardial infarction or accidents.
Of the seven elements studied, magnesium was the only one to show
a pattern of differences completely consistent with the role of
"factor X" in the water story, by being:
1. More abundant in hard than in soft water areas as well as
unaffected by the distribution system (TABLE 9).
2. More abundant in the myocardium of hard water residents
than of soft water residents (TABLE 10).
3. More abundant in the myocardium of healthy subjects than of
those with fatal heart disease.
4. Lastly its concentration in the kitchen tap waters surveyed
yields the strongest association with mortality from all causes
both in Ontario and in Canada (TABLE 11).
TABLE 9
RELATIONSHIP BETWEEN MINERAL CONTENT OF PRIMARY AND TAP WATERS
*----------------------------------------------------------------
Mean Concentration Correlation between
(ppm) Measurement on
Primary Tap Primary and on
Water Water Tap Water
----------------------------------------------------------------
Magnesium 10.62 10.15 0.951
Calcium 33.73 34.20 0.739
Cadmium 0.0005 0.0004 0.054
Zinc 0.18 0.32 0.184
----------------------------------------------------------------
After L. C. Neri et at., 1975.(23)
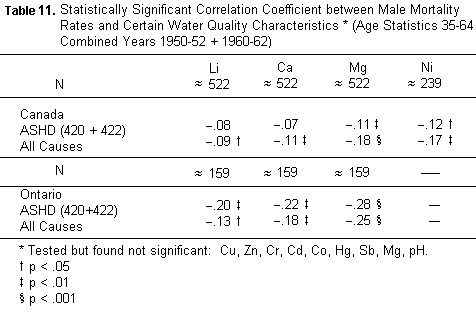
ACKNOWLEDGMENTS
We are very grateful to our colleague Prof. D. Hewitt,
University of Toronto, for his many useful suggestions and for
reviewing the manuscript and to Dr. H. M. Perry, Jr., Washington
University and Dr. J. Marier of the N.R.C. for suggesting the
addition of the section on magnesium.
REFERENCES
1.KOBAYASHI, J. 1957. On geographical relationship between the
chemical nature of river water and death rate from apoplexy. Ber.
Ohara Inst. Landwirtsch. Biol.. Okayama Univ.
11: 12-21.
2.CRAWFORD, M. D. 1972. Hardness of drinking-water and
cardiovascular disease. Proc. Nutr. Soc. 31(3):
347-353.
3.MASIRONI, R. 1973. Water quality, trace elements, and
cardiovascular disease. WHO Chronicle, 1973. 27:
534-538.
4.PUNSAR, S. 1973. Cardiovascular mortality and quality of
drinking water. An evaluation of the literature from an
epidemiological point of view. Work Environ. Health
10: 107-125.
5.NERI, L. C., D. HEWITT & G. B. SCHREIBER. 1974. Can
Epidemiology elucidate the water story. Am. J. Epidemiol.
99: 75.
6.HARNISH, R. A. 1975. Water Hardness and Cardiovascular
disease. Mimeographed article.
7. Müller, G. 1974. Problems of Epidemiological
evaluation of Water Contents. Schriftenr. Ver. Wasser Boden
Lufthyg. Berlin-Dahlem. Stuttgart, 1973. 40:
39-52.
8.NERI, L. C. & D. HEWITT. 1975. Review and implication of
ongoing and projected research outside the European Communities,
Hardness of Drinking Water and Public Health: Proceedings of the
European Scientific Colloquim, Luxembourg. : 443-466. Pergamon
Press. New York.
9.SHARRETT, A. R. & M. FEINLEIB. 1975. Water Constituents
and Trace Elements in Relation to Cardiovascular Disease. Prev.
Med. 4: 20-36.
10.CRAWFORD, M. D., M. J. GARDNER & J. N. MORRIS. 1968.
Mortality and hardness of local water-supplies. Lancet
i: 827-831.
11.LOWE, C. R., C. J. ROBERTS & S. LLOYD. 1971.
Malformations of central nervous system and softness of local
water supplies. Br. Med. J. 2: 357-361.
12.CRAWFORD, M. D., M. J. GARDNER & P. A. SEDGWICK. 1972.
Infant mortality and hardness of local water supplies. Lancet
i: 988-992.
13.ELWOOD, J. M. 1977. Anenchephalus and Drinking Water. Am.
J. Epidemiol. 105: 460-467.
14.SPIERS, P. S., S. G. WRIGHT & D. G. SIEGEL. 1974.
Infant Mortality and Water Hardness in the United States.
Pediatrics 54(3): 317-9.
15.STOCKS, P. 1970. Incidence of Congenital Malformations in
the Regions of England and Wales. Br. J. Prev. Soc. Med.
24: 67-77.
16.SAUER, H. I. 1974. Relationship between trace element
content of the drinking water and chronic diseases, observed
effects of trace metals in drinking water on human health.
Presented at the 16th Water Quality Conference, University of
Illinois, Urbana on February 12, 1974.
17.NERI, L. C., J. S. MANDEL & D. HEWITT. 1972. Relation
between mortality and water hardness in Canada. Lancet
i: 931-934.
18.ANDERSON, T. W., W. H. LERICHE & J. S. McKAY. 1969.
Sudden death and ischemic heart disease. New Engl. J. Med.
280: 805-807.
19.NERI, L. C., D. HEWITT & J. S. MANDEL. 1971. Risk of
sudden death in soft water areas. Am. J. Epidemiol
94: 101-104.
20.SCHROEDER, H. A. 1967. Cadmium, chromium, and
cardiovascular disease. Circulation 35:
570-82.
21.SCHROEDER, H. A. & H. W. VINTON, JR. 1962.
Hypertensioninduced in rats by small doses of cadmium. Am. J.
Physiol. 202: 515-518.
22. SCHROEDER, H. A. 1965. Cadmium as a factor in
hypertension. J. Chronic Dis.18: 647-656.
23. NERI, L. C., H. L. JOHANSEN & F. D. F. TALBOT. 1977.
The chemical content of Canadian drinking water related to
cardiovascular health. Department of Epidemiology, University of
Ottawa and Health and Welfare, Canada.
24. SCHROEDER, H. A. 1966. Municipal drinking water and
cardiovascular death rates. JAMA 1952:
81-85.
25. CRAWFORD, M. D. & T. CRAWFORD. 1969. Lead content of
bones in a soft and hard water area. Lancet I:
699-701.
26. CRAWFORD, M. D. & D. G. CLAYTON. 1973. Lead in bones
and drinking water in towns with hard and soft water. Br. Med. J.
2: 21-23.
27. JOOSSENS, J. V. 1973. Salt and Hypertension: Water
Hardness and Cardiovascular Death Rate, Triangle (Engl Ed)
12(1): 9-16.
28. MORTON, W. E. 1971. Hypertension and drinking water
constituents in Colorado. Am. J. Public Health
61: 1371-1378.
29. FOULGER, J. H. 1943. Medical control of industrial
exposure to toxic chemicals. Ind. Med. 12:
214-225.
30. SYMANSKI, H. 1952. Schwere gesundheitsschadigungen dutch
befufliche nitroglykoleinwirkung. Arch. Hyg. Bakteriol
136: 139-158.
31. FORSSMAN, S., N. MASRELIEZ, G. JOHANSSON, et al. 1958.
Untersuchungen des gesundheitszustandes von nitroarbeitem bei
drei schwedischen sprengstof. fabriken. Arch. Gewerkepathol.
Gewerbehyg. 16: 157-177.
32. SAKADO, A. 1962. A clinical study on nitroglycol
poisoning. Jpn. J. Ind. Health 4: 583. (English
summary, article in Japanese).
33. EINERT, C., W. ADAMS, R. CROTHERS, et al. 1963.
Exposure to mixtures of nitroglycerin and ethylene. glycol
dinitrate. Am. Ind. Hyg. Assoc. J. 24:
435-447.
34. Report of Second Meeting of Investigators on Trace
Elements in Relation to Cardiovascular Disease (WHO/IEAE Joint
Research Programme). Vienna (IAEA) 19-23 February 1973, and
Geneva (WHO) 2-6 April 1973. World Health Org. CVD/73.4.
35.STITT, F. W., M. D. CRAWFORD, D. G. CLAYTON & J. N.
MORRIS. 1973. Clinical and biochemical indicators of
cardiovascular disease among men living in hard and soft water
areas. Lancet i: 122-126.
36.ELWOOD, P. C., D. BAINTON, F. MOORE, D. F. DAVIES, E. J.
WAKLEY, M. LANGMAN & P. SWEETNAM. 1971. Cardiovascular
Surveys in areas with different water supplies. Br. Med. J.
2: 362-363.
37.CRAWFORD, M. D. & J. N. MORRIS. 1967. Lead in Drinking
Water. Lancet ii: 1087-1088.
38.CRAWFORD, T. & M. D. CRAWFORD. 1967. Prevalence and
pathological changes of ischemic heart disease in a hard-water
and in a soft-water area. Lancet i: 229-232.
39.BEEVERS, D. G., E. ERSKINE, M. ROBERTSON, A. D. BEATTIE, B.
C. CAMPBELL, A.GOLDBERG & M. R. MOORE. 1976. Blood Lead and
Hypertension. Lancet ii: 1-3.
40.LANGFORD, H. G., R. L. WATSON & B. H. DOUGLAS. 1968.
Factors affecting blood pressure in population groups. Trans.
Assoc. Am. Physicians 81: 135-146.
41.KEMPER, W. 1944. Treatment of Kidney Disease and
Hypertensive Vascular Disease with Rice Diet. N. C. Med. J.
5: 125-133.
42.MENEELY, G. R. 1967. The Experimental Epidemiology of
Sodium Chloride Toxicity in the Rat. In The Epidemiology
of Hypertension. J. Stamber, R. Stamier, T. N. Pullman, Eds. :
240. Grune & Stratton. New York & London.
43.SHAPER, A. G., P. J. LEONARD, K. W. JONES & M. E.
JONES. 1969. Environmental effects on the body build, blood
pressure and blood chemistry of nomadic warriors serving in the
army in Kenya. Afr. Med. J. 46: 282-286.
44. SAKAKI, N. 1964. The relation of salt intake to
hypertension in the Japanese. Geriatrics 19:
735-744.
45. STEINBACH, M., M. CONSTANTINEANU & P. NARNAGEA. 1975.
Relationship between the genetic and the ecologic factors in the
determination of high blood pressure. Rev. Roum. Med.
13(4): 261-263.
46.FODOR, J. G., A. C. ABEOTT & I. E. RUSTED. 1973. An
epidemiological study of hypertension in Newfoundland. Can. Med.
Assoc. J. 108: 1365-13,68.
47. FODOR, I. G. & I. E. RUSTED. Salt and Hypertension.
Paper presented at the American Heart Assoc. Meeting, February
1976, New Orleans.
48. SCHROEDER, H. A. 1960. Relation between mortality from
cardiovascular disease and treated water supplies: variations in
states and 163 largest municipalities of the United States. JAMA
172: 1902-1908.
49. LEWIS, M. 1974. Letter: Softened Water. JAMA
228(8): 978.
50. PERRY, H. M. 1973. Minerals in cardiovascular disease. J.
Am. Dietetic Assoc. 62: 631-637.
51.PERRY, H. M., JR. & E. F. PERRY. 1974. Possible
relationships between the physical environment and human
hypertension: cadmium and hard water. Prev. Med.
3: 344-352.
52.PERRY, H. M., G. S. THIND & E. F. PERRY. 1976. The
Biology of cadmium Med. Clin. North Am. 60(4):
759-69.
53.PERRY, H. M., JR., M. W. ERLANGER & E. F. PERRY. 1974.
Prevention of cadmium-induced hypertension by selenium,.,zinc,
and hard water. 8th Annual Conference on Trace Substances in
Environmental Health, Columbia, Mo.
54.THIND, G. S. 1972. Role of cadmium in human and
experimental hypertension. J.Air Pollut. Control Assoc.
22(No.4): 267-270.
55.LENER, J. B. 1971. Cadmium and hypertension. Lancet
i: 970.
56.THIND, G. S. & O. M. FISCHER. 1974. Relationship of
plasma zinc to human hypertension. Clin. Sci. Mol. Med.
46: 137-141.
57.VOORS, A W., M. S. SHUMAN & P. N. GALLAGHER. 1972. Zinc
and cadmium; autopsy levels for cardiovascular disease in
geographical context. 6th Annual Conference on Trace . Substances
in Environmental Health.-Columbia, Mo.
58.BIERENBAUM, M. L., A. I. FLEISCHMAN, J. DUNN & J.
ARNOLD. 1975. Possible toxic water factor in coronary heart
disease. Lancet I: 1008-1010.
59.SHARRETT, A. R. & M. FEINLEIB. 1975. Possible toxic
water factor in coronary heart disease. Lancet
ii: 76.
60.CARROLL, R. E. 1966. The relationship of cadmium in the air
to cardiovascular disease death rates. JAMA 198:
267-269.
61.TSUCHIYA, K. 1967. Proteinuria of workers exposed to
cadmium fumes. Arch. Environ. Health 14:
876-880.
62.HAMMER, D. I., J. F. FINKLEA, J. P. CREASON, S. H.
SANDIFER, J. E. KEIL, L. E. PRIESTER & J. F. STARA. 1971.
Cadmium exposure and human health effects. In Trace Substances in
Environmental Health V. D. D. Hemphill, Ed. : 269-283. University
of Missouri Press. Columbia, Mo.
63. Blood Pressure of Adults by Race and Area, United States
1960-62. Vital and Health Statistics Series 11 No. 5. U.S. Dept.
of Health Education and Welfare.
64. Hypertension and Hypertensive Heart Disease in Adults,
United States 1960-62. Vital and Health Statistics Series 11, No.
13. U.S. Dept. of Health Education and Welfare.
65. Muss, D. L. 1962. Relation between water quality and
deaths from cardiovascular disease. J. Am. Water Works Assoc.
54(11): 1371-1378.
66. STÖFFN, D. 1974. Environmental lead and the heart. J.
Mol. Cell Cardiol. 6: 285-90.
67. MOORE, M. R., P. A. MEREDITH, A. GOLDBERG, K. E. CARR, P.
G. TONER & T.D. V. LAWRIE. 1975. Cardiac effects of lead in
drinking water of rats. Clin. Sci. Mol. Med. 49:
337-341.
68. NERI, L. C., D. HEWITT, G. B. SCHREIBER, T. W. ANDERSON,
J. S. MANDEL & A. ZDROJEWSKY. 1975. Health aspects of hard
and soft water. J. Am. Water Works Assoc. 67(8):
403-409.
69. SIX, K. M. & R. A. GOYER. 1973. Experimental
enhancement of lead toxicity by low dietary calcium. J. Lab.
Clin. Med. 76: 933-942.
70. HARTUNG, R. 1973. The biological effects of heavy metal
pollutants in water. In Proceedings of a Conference on
the Role of Metal Ions in Biological Systems, November 20-21,
1972 Argonne, Illinois. Plenum Press. New York.
71. SCHROEDER, H. A. 1965. The biological trace elements. J.
Chronic Dis. 18: 217-228.
72. SCHROEDER, H. A. & L. A. KRAEMER. 1974. Cardiovascular
mortality, municipal water and corrosion. Arch. Environ. Health
28: 303-311.
73. NERI, L. C. 1970. The relationship between water hardness
and cardiovascular mortality (The Canadian Experience). Thesis,
University of Toronto. Toronto, Ont., Canada.
74. SCHROEDER, H. A. 1966. Municipal drinking water and
cardiovascular death rates. JAMA 195(2):
81-85.
75. SAUER, H. I., D. W. PARKE & M. L. NEILL. 1970.
Associations between drinking water and death rates. In
Trace Substances in Environmental Health IV. D.D. Hemphill, Ed. :
318. University of Missouri Press. Columbia, Mo.
76. CRAWFORD, M. D., M. J. GARDNER & J. N. MORRIS. 1968.
Mortality and hardness of local water-supplies. Lancet
i: 827-831.
77. LEWIS, G. P., W. J. JUSKO, L. L. COUGHLIN & S. HARTZ.
1972. Cadmium accumulation in man: influence of smoking
occupation, alcoholic habit and disease. J. Chronic Dis.
25: 717-726.
78. MASIRONI, R. 1970. Cardiovascular mortality in relation to
radioactivity and hardness of local water supplies in the U.S.A.
Bull. WHO 43: 687-697.
79. MASIRONI, R., S. R. KOIRTYOHANN, J. O. PIERCE & R. G.
SCHAMSCHULA. 1976. Calcium content of river water, trace element
concentrations in toenails, and blood pressure in village
populations in New Guinea. Sci. Total Environ.
6: 41-53.
80.SEELIG, M. S. & H. A. HEGGTVEIT. 1974. Magnesium
interrelationships in Ischemic Heart Disease: A review. Am. J.
Clin. Nutr. 27: 59-79.
81. SZELENYI, I. 1973. Magnesium and its significance in
Cardiovascular and Gastro-Intestinal disorders. World Rev. Nutr.
Diet. 17: 189-224.
82.MARIER, J. R. 1976. Some current thoughts on magnesium with
particular emphasis on the water-factor and cardiac disorders.
Mimeographed monograph.
83.ISERI, L. T., J. FREED & A. R. BURES. 1975. Magnesium
deficiency and cardiac disorders. Am. J. Med.
58: 837-846.
84.BAJUSZ, E. 1965. In Nutritional Aspects of
Cardiovascular Disease. : 129-131. Crosby Lockwood. London.
85.HEROUX, O., D. W. PETER & H. A. HEGGTVEIT. 1973.
Potentiation of cold induced cardiac lesions in white rats
resulting from chronic dietary magnesium insufficiency.
In First International Symposium on Magnesium Deficit in
Human Pathology. J. Durlach, Ed.: 337, 341. Springer-Verlag.
86.SHUSTVOL, N. S. & A. A. BERESTOV. 1973. The effects of
potassium chloride and magnesium chloride in electrocardiographic
and morphologic changes in ischemic necrosis of the myocardium.
Patol. Fiziol. Eksp. Ter. 17; 19-23.
87.MALVIEL-SHAPIRO, B. 1958. Further observations on
parenteral magnesium sulphate therapy in coronary heart disease.
S. Afr. Med. J. 32: 1211-1214.
88.PARSONS, R. S., T. L. BUTLER & E. P. SELLARS. 1960.
Coronary artery disease. Med. Proc. 6:
479-481.
89.THURNHERR, A. & J. KOCH. 1962. Versuch einer
Kausaltherapie der Arteriosclerose mit einer Kombination von
Hyaluronidase und Magnesium. Schweiz. Med. Wochenschr.
92: 949-956.
90.SEELIG, M. S. 1964. The requirements of magnesium by the
normal adult. Am.J Clin. Nutr. 14: 242-290.
91.MARIER, J. R. 1968. The importance of dietary magnesium
with particular reference to humans. Vitalst. Zivilisationskr.
13: 144-149.
92.SCHROEDER, H. A. 1971. Losses of vitamins and trace metals
resulting from processing and preservation of food. Am. J. Clin.
Nutr. 24: 562-573.
93.BROWN, J. O. & 18 others. 1970. Nutritional and
epidemiologic features related to heart disease. World Rev. Nutr.
Diet. 12: 1-43.
94.ANDERSON, T. W., L. C. NERI, C. B. SCHREIBER, F. D. F.
TALBOT & A. ZDROJEWSKI. 1975. Ischemic heart disease, water
hardness and myocardial magnesium. Can. Med. Assoc: J.
113L 188-203.
This page was first uploaded to The Magnesium Web Site on
August 29, 1996
http://www.mgwater.com/