Journal of Nutritional & Environmental Medicine
(1999) 9, 5-13
EDITORIAL
Is ISIS-4 research misconduct?
DAMIEN DOWNING MBBS
BSAENM, PO Box 3AP, London W1A 3AP, UK
INTRODUCTION
For 40 years now, researchers have been claiming that
parenteral magnesium administration may have a beneficial effect
on mortality and morbidity in acute myocardial infarction (AMI)
[1, 2]. For about the same time, an association has been apparent
between magnesium deficiency and prevalence of ischaemic heart
disease [3, 4]. By 1992 several small controlled trials had shown
a positive result for magnesium therapy in AMI [5-12], and a
meta-analysis concluded that it reduced short-term mortality more
than any other agent [15]. The same year, the LIMIT-2 study
confirmed this with a 24% reduction in mortality in the first 28
days [17] (and 21% in the longer term [18]). Then, in 1995, the
very large-scale ISIS-4 study appeared, reporting no benefit from
magnesium, and indeed a slight detriment [20]. Very quickly, this
study was used to support the argument that magnesium offered no
benefit in AMI [21]. and, further, that meta-analysis is
intrinsically flawed, and only mega-trials will lead us to the
truth [22].
However, there are several clear methodological flaws in the
ISIS-4 protocol which call into question both its finding on
magnesium, and the more general conclusion that has been drawn
concerning mega-trials. The implications of these shortcomings
are evidently serious for AMI sufferers, but also therefore for
the progress of medical science at large. They also inescapably
raise the question of whether ISIS-4 is an instance of
misreporting that amounts to research misconduct.
History of Mg in IHD
In 1958, Malkiel-Shapiro reported that magnesium injections
appeared to be life-saving in acute myocardial infarction. Sixty-
four patients with documented AMI or acute coronary insufficiency
were treated with a series of intramuscular magnesium injections
(there were no controls). Only one of the patients died within
4-6 weeks of the attack (1.6% mortality), compared to an
anticipated average mortality of 19-50% [1].
Similar results were reported a year later by Parsons et
al. In that study, 100 patients with acute coronary heart
disease, of which at least one-third had AMI, received a series
of intramuscular injections of magnesium sulphate. Only one death
occurred—a mortality rate of 1%—compared to a
mortality of about 30% in controls not given magnesium [2].
In the 1980s a number of small, controlled studies appeared,
showing a lower mortality and/or a reduced rate of arrhythmias in
the magnesium-treatment groups [5-12]. Although the total number
of patients studied in these papers was small (just over 1000
altogether), they did, both individually and when grouped, show a
significant benefit from magnesium [17]. There were 2 studies
during that period that showed negative results. Abraham [13],
which used the lowest Mg dose of any of this set of studies,
showed a reduction in tachyarrhythmias, but no change in other
cardiac parameters or in mortality. Feldstedt [14] used the
highest Mg dose, and found an increase in bradyarrhythmias, heart
failure and mortality.
Indeed, both a 1992 meta-analysis [15] and a 1991 overview
[16] concluded that there was strong evidence from these studies
for a benefit from magnesium. The overview had 5 co-authors, of
whom 3 were later on the steering committee of ISIS-4.
In 1992 the LIMIT-2 (second Leicester Intravenous Magnesium
Intervention Trial) report appeared [17]. This study randomized
2316 patients, and used a protocol of MgSO4 73mmol IV in the
first 24 hours, with start of treatment averaging 3 hours from
onset of symptoms. The overall reduction in mortality was
24%.
Then, in November 1993, the results of the extremely large
(58,050 patients in 1086 hospitals) ISIS-4 multi-centre study
were ‘leaked’ at a cardiology conference in the USA.
These appeared to show no benefit for magnesium, and even perhaps
a slight worsening of survival. Several months later the
Lancet was commenting on why these had not yet appeared
in print, and a few months after that they were saying that the
result as regards magnesium had already been called into question
because of methodological shortcomings in the ISIS-4 study. Then,
in March 1995, the Lancet finally published the results
[20].
Although the ISIS-4 mega-trial has been put forward as a
demonstration that magnesium lacks any benefit in acute
infarction, it could equally be argued that it clarifies the
mechanism involved, and indicates how and when it should be
administered. This did not deter the BMJ from
publishing, the following week, two editorials both stating that
meta-analysis must be regarded as flawed, that the truth will
only emerge from mega-trials, and that we now know ‘the
truth’ about magnesium—that it is ineffective [21,
22]. In retrospect this begins to appear over-hasty to say the
least.
The flaws in ISIS-4
There are several major criticisms that have been made of the
ISIS-4 protocol, and although some elucidatory research has been
done since it appeared, much of the literature that validates
these criticisms was available before ISIS-4 was
implemented—indeed it is largely cited by the ISIS-4 team
members in the 1991 overview mentioned above [16]. The principal
methodological criticisms are that the administration of
magnesium was:
Too much
The beneficial cardiovascular effects of magnesium include a
lowering of blood pressure through vasodilatation, a slowing of
the heart rate, and reduced excitability of myocardium. Excessive
dosages will cause harmful hypotension and bradycardia, and
possibly heart block and failure. In ISIS-4, hypotension was more
frequent in patients given magnesium, as were heart block and
heart failure, and cardiogenic shock. These findings are very
similar to—and indeed could have been predicted
from—those in the study by Feldstedt et al. [14],
but were not seen in any of the other studies. The small studies
in the 1980s and early 1990s [5-14] used a wide range of doses of
magnesium, almost all of them between 32 and 73 mmol in the first
24 hours (Fig. 1). The notable exceptions were the studies by
Abraham et al. [13] which employed 10 mmol and reported
strictly limited effects, and the Feldstedt et al. paper
which used 80 mmol and showed changes consistent with magnesium
toxicity.
In ISIS-4 a total of 80 mmol of Mg was administered in the
first 24 hours, which is more than in any other study, but
exactly the same as that used in Feldstedt. Figure 1 (modified
from Seelig and Elin [24]) plots the outcome, in terms of
percentage change in mortality, against Mg dosage, in published
studies up to and including ISIS-4. The addition of the LIMIT and
ISIS-4 papers to those published before 1991, when ISIS-4 began
data gathering, does not significantly alter the best-fit curve
to be derived from these data points, which show that,
unsurprisingly, it is possible to overdose magnesium.
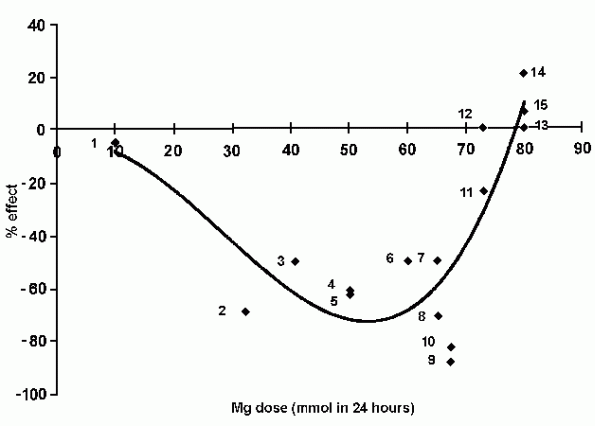
|
Fig. 1
|
Paper
|
Mg dose
|
% effect
|
Year
|
Reference
|
|
1
|
Abraham
|
10
|
25
|
1987
|
13
|
|
2
|
Ceremuzynski
|
32
|
-69
|
1989
|
12
|
|
3
|
Bertschat
|
40.5
|
-50
|
1989
|
10
|
|
4
|
Rasmussen
|
50
|
-61
|
1986
|
6
|
|
5
|
Thorgersen
|
50
|
-63
|
1995
|
39
|
|
6
|
Morton
|
60
|
-50
|
1984
|
5
|
|
7
|
Singh
|
65
|
-50
|
1990
|
11
|
|
8
|
Smith
|
65
|
-71
|
1986
|
8
|
|
9
|
Schecter
|
67
|
-88
|
1990
|
9
|
|
10
|
Schecter
|
67
|
-83
|
1990
|
9
|
|
11
|
LIMIT-2
|
73
|
-24
|
1992
|
17
|
|
12
|
Barghava
|
73
|
0
|
1995
|
40
|
|
13
|
Flather
|
80
|
0
|
1991
|
|
|
14
|
Feldstedt
|
80
|
20
|
1991
|
14
|
|
15
|
ISIS-4
|
80
|
6
|
1995
|
20
|
FIG. 1. Outcome in published studies up to and including
ISIS-4.
The ISIS-4 published protocol document [19] contains no
discussion of the choice of magnesium dosages, nor any rationale
for the injudiciously high dosage used. Dosage-finding studies
were conducted, and cited, for captopril and mononitrate [25,
26], but not for magnesium. The steering committee of ISIS-4
clearly knew about the studies that make up the above
curve—several of them put their name to a 1991 overview of
them [17]. By choosing a dose of magnesium that had previously
been shown to produce toxicity signs and an increase in
mortality, the designers appear to have put patients at risk, and
probably a substantial number of patients died as a direct
result. At best, they must be open to accusations of wilful
carelessness in the dosage selection for one of their
interventions—but only for one of the three.
Too late
Magnesium protects against many of the complications that can
occur in acute MI, but it is well established that, to be
effective, administration should begin as soon as possible after
the onset of symptoms. This becomes particularly important when
thrombolysis is performed, because successful thrombolysis
re-establishes blood-flow to damaged myocardium, and the
consequent reperfusion injury can itself be harmful. Magnesium
protects against this, but only if it is given early enough.
Epidemiological and laboratory studies indicate that low
tissue magnesium levels can contribute to the development of
coronary heart disease [27-29]. When AMI occurs, magnesium levels
in myocardium fall dramatically, allowing an influx of calcium
into cells, leading to increased risk of arrhythmia and to
potentiation of irreversible cell damage [30-32]. Magnesium
administered parenterally protects against these injuries to the
heart and their complications; this was clearly demonstrated by
the series of studies between 1980 and 1995 cited above [5-14],
none of which (excluding the LIMIT-2 study) involved the use of
thrombolysis.
When, as in the LIMIT and ISIS-4 studies, thrombolysis is
performed, a further therapeutic role for magnesium is called
into play. Reperfusion injury describes a sequence of events that
result from restoration of blood supply to infarcted areas of
myocardium, which includes damage mediated by free-radicals and
by calcium overload [33]. The consequences include myocardial
stunning and arrhythmias, and the death of previously viable
cells. Magnesium, given in appropriate doses and with appropriate
timing, protects against these effects also [34-37].
In streptokinase thrombolysis, achieving 50-60% restoration of
arterial patency takes approximately 90 minutes from the
beginning of thrombolysis (a figure cited in the full ISIS-4
paper). As soon as blood flow is re-established, reperfusion
injury will start to occur. For magnesium to protect against
this, it needs to be present in effective concentration before
this time; it therefore needs to be administered early enough. In
a proportion of unthrombolysed patients, spontaneous reperfusion
will nevertheless occur during the first few hours after AMI,
with reperfusion injury resulting from this also. Timing of
magnesium therapy in relation to both reperfusion and onset of
symptoms is therefore important. The crucial importance of timing
was examined thoroughly in four animal studies [35-38] and one
further human study [39], all of which appeared in 1995, so were
not available to the ISIS-4 researchers. Nonetheless, it does not
take a scientist to appreciate that if a therapeutic agent is not
present in the body when a harmful event occurs it cannot
moderate the effects of that event. In summary, the clear finding
of these studies was that delaying the start of magnesium therapy
to 1 hour after the start of thrombolysis removed or severely
diminished the protective effect of magnesium.
In the LIMIT-2 study [17,18], the median time from onset of
symptoms to randomization was 3 hours. Allocation to thrombolysis
and/or magnesium therapy then occurred simultaneously, so that
magnesium was always given early, and begun no later than
thrombolysis. The reduction in 28-day mortality for those
allocated to magnesium vs controls was 24%. In ISIS-4 the median
time from onset of symptoms to randomization was 8 hours, and a
maximum gap of 24 hour s from onset of symptoms to magnesium (or
other) therapy was set. Moreover, thrombolysis was required to be
started before randomization, and therefore magnesium therapy
could take place. The ISIS-4 paper states: "For patients
receiving both fibrinolytic and magnesium infusions, the
magnesium was begun within 2 h of the start of the fibrinolytic
in about half of all randomized patients" [20]. The finding of
the ISIS- 4 study was that magnesium conferred no protection.
Although both the ISIS-4 paper and Yusuf and Flather, in their
BMJ editorial [21], have argued that reperfusion would
have been almost simultaneous with magnesium in a large
proportion of patients, it is in fact clear that considerably
more than half of the magnesium-allocated patients would not have
received it until after the 1-hour point. Given that the protocol
stated that "Study treatment was generally to be started
immediately after the early lytic phase (i.e. the first hour or
so) of any fibrinolytic regimen", it further seems clear that in
respect of magnesium administration the protocol was violated in
over 50% of ISIS- 4 subjects [42]. As Woods succinctly stated in
his 1995 review of the subject [23]; "The study was not designed
to test for an effect of magnesium on reperfusion injury and
cannot retrospectively be made to do so." Equally, given the
permitted delay between onset of symptoms and start of therapy,
it was not designed to test for an effect of magnesium on
ischaemic injury either.
Too quick
Apart from the effects of magnesium on myocardial damage and
mortality from ischaemia and reperfusion, there is a separate
direct effect on the risk of tachyarrhythmias. This has been
shown to operate both in AMI and in other, non-cardiac, disorders
[43, 44]. Tachyarrhythmias can occur immediately, as a result of
ischaemia and/or reperfusion, or later on for a variety of
reasons. The need to maintain magnesium levels does not cease
after the first few hour s of infarction, therefore.
The earliest studies of Mg in heart disease [1,2] used
intramuscular injections. Those published between 1980 and 1991
[5-14] used intravenous infusions with or without initial slow
intravenous ‘bolus’ injections, treatment lasting 24
hours in three of these [8,12, 14], 36 hours in one [5] and 48 or
72 hours in the remainder. Of the 24-hour studies two showed a
substantial decrease in mortality, the exception being Feldstedt,
which used the same ‘overdosage’ of magnesium as
ISIS-4. All of the 36-72 hour studies showed an improvement in
mortality. The two subsequent studies, LIMIT and ISIS-4, both
limited magnesium administration to 24 hours; neither found an
improvement in the rate of arrhythmias requiring treatment.
No laboratory or human studies have since been conducted to
determine the importance of magnesium duration (in contrast to
the timing of magnesium onset), nor has the timing of arrhythmias
in relation to magnesium therapy in the studies cited been
accessible to post-hoc analysis. At present, therefore, we can
only say that this aspect of magnesium therapy requires further
study to achieve certainty. When Teo et al. [16]
reviewed the effects of magnesium in 1991, however, 4 of the 7
trials that they considered had given magnesium for more than 24
hours, and none for less. Apart from Feldstedt all had used a
smaller dosage of magnesium than ISIS-4 did. All but one of the 7
studies showed a reduction in arrhythmias. The ISIS-4 protocol
gave no reason for choosing, from the range of previous study
models available to them, the maximum dose of magnesium, given
over the minimum duration of infusion.
Too uncontrolled
In contrast to the assertion by several theorists that ISIS-4
amounts to conclusive evidence that large-scale trials are
necessary to establish the truth regarding treatment effects,
outweighing meta-analysis and demonstrating its shortcomings, the
very validity of the study as a mega-trial has been questioned.
The point has been made that ISIS-4 was conducted in 1086
hospitals in 31 countries—an average of 53 patients per
hospital. Inevitably, there will have been wide variations in
social and physical settings between hospitals, leading to wide
variations in implementation of the study protocol—and thus
to a reduction in the homogeneity of the results. Given this, it
has been suggested that ISIS- 4 could best be regarded as 1000 +
small trials rather than one large one. As such, the appropriate
statistical models for analysis of the results would be
different. Antman [42, 45] observed that, for examining pooled
data from multiple studies, the fixed effects model assumes
homogeneity among the trials, whereas the random effects model
allows for variability between them. The most noticeable
difference between these two models is typically that the
confidence intervals for the treatment effects are greater with
the random effects model. Applying this comparison of statistical
tools to the studies up to and including LIMIT, ISIS-4 and the
1995 trial by Schecter [46] (looking at the effect of magnesium
in subjects considered unsuitable for thrombolytic therapy),
Antman found an odds ratio for magnesium vs control of 1.02
(0.96-1.08) by the fixed effects model but 0.59 (0.39-0.90) by
the random effects model—a striking difference. Were such
an analysis to be utilized for an internal analysis of the
results of ISIS-4, the conclusions might have been very
different.
A similar point applies to the consideration of individual
patients in large-scale trials; as Woods states, "the simplicity
of the design imposes a reductionist model on any treatment
effect—one size fits all" [23]. Readers of this journal
more than most are likely to appreciate this point concerning the
individuality of patients, and the reductionist effect of fitting
all of them, regardless of treatment needs, into a rigid
randomization protocol. Some studies since ISIS-4 have begun to
look at the possibility that magnesium might be appropriate for
sub-sets of AMI patients [46-48]. The use of different study
models, together with appropriate statistical methods based on
heterogeneity rather than assuming homogeneity, offers hope of
identifying such groups of patients and serving them better in
future.
But the most interesting such observation to emerge from
analysis of ISIS-4 and the other studies relates to the influence
on treatment effects of control group mortality. This underlying
mortality may be influenced by a variety of factors—in many
studies on AMI, for instance, non-trial nitrate therapy is
allowed at the discretion of treating doctors, without resulting
in exclusion from the trial. Indeed it is clear that ethical
considerations would make it difficult to withhold such measures
for the sake of a study. In ISIS-4 60% of patients received such
non-trial treatment. The unsurprising finding, in general, is
that the lower the control mortality, the less benefit can be
derived from treatment—otherwise we would expect negative
mortality rates in treated groups—and the greater the size
of study groups needed to establish any treatment effect.
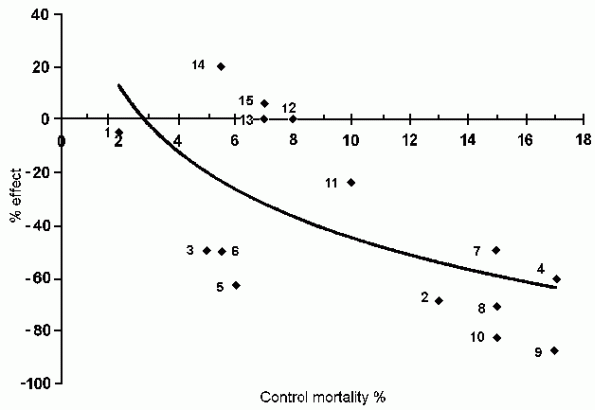
In Fig. 2, the magnitude of treatment effect is plotted
against control mortality for 15 studies including ISIS-4; the
curve (in this case a logarithmic trendline) crosses the zero
line at about 3% control mortality. In a similar analysis Antman
[42] found that the regression curve for treatment effect reached
zero at a control mortality of approximately 7%. A reasonable
inference might be that there is an inescapable—and so far
untreatable, by magnesium or anything else—mortality rate
from AMI of around 5%, which is borne out by the average
treatment group mortality in all these studies of 4.9%.
The consequence for the ISIS-4 study, with a control mortality
of about 7%, is that despite its large size it was a poor
instrument for detecting treatment effects. While this would also
appear to have been the case, to a lesser degree, for captopril
and mononitrate, it was especially true for magnesium due to the
factors discussed above.
Conclusions
Considering all these factors together, it appears that the
effect in the case of ISIS-4 has been to produce a classical Type
II error. This goes against conventional statistical wisdom,
which says that the only real protection against such errors lies
in large numbers. It has further led to a shift in the consensus
view of meta-analysis, based on an acceptance that ISIS-4 was
right, and the clear inference that meta-analysis is therefore
fundamentally flawed. This must now be questioned.
The lack of discussion of magnesium treatment choices in
either the ISIS-4 protocol paper or the full report makes it
impossible to draw any conclusions regarding the thinking or
motives of the steering committee. But the outcome of ISIS-4 does
have to be regarded as a good one for the researchers (evidence
that large-scale studies work, and that many more will be needed)
and for the principal funders (evidence that captopril works, but
mononitrate and magnesium do not).
|
Fig. 2
|
Paper
|
Control
|
Treatment
|
| |
|
Mortality %
|
Effect as %
|
|
1
|
Abraham
|
2
|
25
|
|
2
|
Ceremuzynski
|
13
|
-69
|
|
3
|
Bertschat
|
5
|
-50
|
|
4
|
Rasmussen
|
17
|
-61
|
|
5
|
Thorgersen
|
6
|
-63
|
|
6
|
Morton
|
5.5
|
-50
|
|
7
|
Singh
|
15
|
-50
|
|
8
|
Smith
|
15
|
-71
|
|
9
|
Schecter
|
17
|
-88
|
|
10
|
Schecter [41]
|
15
|
-83
|
|
11
|
LIMIT-2
|
10
|
-24
|
|
12
|
Barghava [40]
|
8
|
0
|
|
13
|
Flather
|
7
|
0
|
|
14
|
Feldstedt
|
5.5
|
-20
|
|
15
|
ISIS-4
|
7
|
6
|
FIG. 2. The magnitude of treatment effect plotted against
control mortality for the 15 studies culminating in ISIS-4.
Every major study these days involves a press release
announcing the results. These are the scientific equivalent of
the work of ‘spin doctors’ in government. A further
cause for concern regarding ISIS-4 is the way in which the
results were released, in November 1993, in the form of a press
release embargoed until the first day of the American Heart
Association meeting in Atlanta, at which the preliminary results
were presented to the meeting. At this time, according to the
final paper (which appeared somewhat later, in March 1995), it
was only 9 weeks since randomization had ended, and only 81% of
follow-up data was available for analysis. Already though, the
tone of the press release makes it clear how the authors wished
their report to be viewed: Dr Rory Collins, the ISIS- 4
coordinator, said "These results will disappoint those who had
trusted the positive claims from previous small trials of
magnesium and nitrates."
In an editorial in 1991 we discussed the issue of
‘Tabloid Science’: ‘the public, bombarded with
health stories alongside politics, sport and the rest of the
news, have no opportunity to acquire an overview of the research
evidence; each story is displaced from memory by the next, or the
one after that, and perception of the truth is ephemeral,
immediate and partial, and often trivialized’ [49]. In this
context, the combination of the ISIS-4 report and the way it was
presented to the world looks like a deliberate and so far very
successful strategy. Those who had trusted the previous studies
were indeed disappointed, though not necessarily in the way meant
by Dr Collins, and the impact on clinical care has been
substantial. If they are not deliberate, then the multiple
anomalies in the design and execution of ISIS-4, whose effects
all accumulate towards an underestimate of the usefulness of
magnesium, must indicate startling ignorance and/or negligence on
the part of the ISIS- 4 authors. Either this was bungling or it
was research misconduct.
REFERENCES
[1] Malkiel-Shapiro B. Further observations on parenteral
magnesium sulfate therapy in coronary heart disease: a clinical
appraisal. S Afr Med J 1958; 32: 1211.
[2] Parsons RS, et al. The treatment of coronary
artery disease with parenteral magnesium sulfate. Med Proc 1959;
5: 487-98.
[3] Seelig M.S. Magnesium deficiency in the pathogenesis of
disease. In: Plenum Medical Book, New York and London 1980.
[4] Morgan K.J., Stampley G.L., Zabik M.E., Fischer D.R.:
Magnesium and Calcium Dietary Intakes of the U.S. Population. J
Am Coll Nutr 1985; 4: 195-206.
[5] Morton BC, Smith FM, Nair RC, McKibbon TG, Poznanski WJ.
Magnesium therapy in acute myocardial infarction: a double blind
study. Magnesium 1984; 3: 346-52.
[6] Rasmussen HS, McNair P, Norregard P, Backer V, Lindeneg O,
Balslev S. Intravenous magnesium in acute myocardial infarction.
Lancet 1986; 1: 234-6.
[7] Rasmussen HS, Gronbaek M, Cintin C, Balslev S, Norregard
P, McNair P. One-year death rate in 270 patients with suspected
acute myocardial infarction, initially treated with intravenous
magnesium or placebo. Clin Cardiol 1988; 11: 377-81.
[8] Smith LF, Heagerty AM, Bing RF, Barnett DB. Intravenous
infusion of magnesium sulphate after acute myocardial infarction:
effects on arrhythmias and mortality. Int J Cardiol 1986; 12:
175-80.
[9] Shechter M, Hod H, Marks N, Kaplinsky E, Behar S,
Rabinowitz B. Beneficial effect of magnesium sulfate in acute
myocardial infarction. Am J Cardiol 1990; 66: 271-4.
[10] Bertschat F, Ising H, Gilnther T, et al.
Antiarrhythmic effects of magnesium infusions in patients with
acute myocardial infarction. Magnesium Bull 1989; ii: 155-8.
[11] Singh RB, Sircar AR, Rastogi SS, Garg V, Magnesium and
potassium administration in acute myocardial infarction Magnes
Trace Elem 1990; 9: 198-204.
[12] Ceremuzynski L, Jurgiel R, KulakowskiP, Gebalska J.
Threatening arrhythmias in acute myocardial infarction are
prevented by intravenous magnesium sulphate. Am Heart J 1989;
118: 1333-4.
[13] Abraham AS, Rosenmann D, Kramer M, et al.
Magnesium in the prevention of lethal arrhythmias in acute
myocardial infarction. Arch Intern Med 1987; 147:753-5
[14] Feldstedt M, Boesgaard S et al. Magnesium
substitution in acute ischaemic heart syndromes. Eur Heart J
1991; 12: 1215-8.
[15] Lau J., Elliott M., Antman M.D., Jimenez-Silva J.,
Kupelnick B., Mosteller F., Chalmers T.C., Cumulative
Meta-Analysis of Therapeutic Trials for Myocardial Infarction. N
Engl J Med 1992; 327(4): 248-54.
[16] Teo KK, Yusuf S, Collins R, Held PH, Peto R. Effects of
intravenous magnesium in suspected acute myocardial infarction:
overview of randomised trials. BMJ 1991; 303: 1499-503.
[17] Woods KL, Fletcher S, Roffe C, Haider Y. Intravenous
magnesium sulphate in suspected acute myocardial infarction:
results of the second Leicester Intravenous Magnesium
intervention Trial (LIMIT-2). Lancet 1992; 339: 1553-58.
[18] Woods K.L., Fletcher S. Long-term outcome after
intravenous magnesium sulphate in suspected acute myocardial
infarction: the second Leicester Intravenous Magnesium
intervention Trial (LIMIT-2). Lancet 1995; 343: 816-9.
[19] ISIS-4 Collaborative Group. Fourth International Study of
Infarct Survival: Protocol for a Large Simple Study of the
Effects of Oral Mononitrate, of Oral Captopril, and of
Intravenous Magnesium. Am J Cardiol 1991; 86: 87D-100D.
[20] ISIS-4 Collaborative Group. ISIS-4: a randomized
factorial trial assessing early oral captopril, oral mononitrate,
and intravenous magnesium sulphate in 58,050 patients with
suspected acute myocardial infarction. Lancet 1995; 345:
669-85.
[21] Yusuf S, Flather M. Magnesium in acute myocardial
infarction (Editorial). BMJ 1995; 310: 751-2.
[22] Egger M, Davey Smith G. Misleading meta-analysis
(Editorial). BMJ 1995; 310: 752-4.
[23] Woods KL. Mega-trials and management of acute myocardial
infarction. Lancet 1995; 346: 611-14.
[24] Seelig MS, Elin RJ. Is there a place for magnesium in the
treatment of acute myocardial infarction? Am Ht J 1996; 132:
471-76.
[25] Conway M, Mohideen R et al. A dose-finding study
for the initiation of captopril within 24 h of acute myocardial
infarction. (Pre-pilot study for ISIS-4). Q J Med. 1989; 70-73:
973- 4.
[26] Pipilis A, Flather M, Collins R, Conway M, Sleight P.
ISIS-4 pilot study: serial haemodynamic changes with oral
captopril and oral isosorbide mononitrate in a randomised
double-blind trial in acute myocardial infarction. J Am Coll.
Cardiol 1991; 17: 115A.
[27] Rayssiguier Y. Magnesium and Lipids Interrelationships in
the Pathogenesis of Vascular Diseases. Magnesium-Bulletin 1981;
3: 165-77.
[28] Durlach J. Pilule et Thrombose; ou des Plaquettes, des
Estrogenes et du Magnesium. Revue Francais Endocrinologie
Clinique 1970; 11: 45-54.
[29] Haddy FJ, Seelig MS. Magnesium and the Arteries. II.
Physiologic Effects of Electrolyte Abnormalities in Arterial
Resistance. In: Magnesium in Health and Disease. Eds. M. Cantin
& M.S. Seelig. Publ. SP Medical & Scientific Books, New
York. 1980; 639-57.
[30] FIink EB, Brick JE, Shane SR. Alterations of long-chain
free fatty acid and magnesium concentrations in acute myocardial
infarction Arch Intern Med 1981;141:44l-3.
[31] Reimer KA, Lowe JE, Rasmussen MM, et al. The
wave-front phenomenon of ischaemic cell death 1: Myocardial
infarct size vs duration of coronary occlusion in dogs.
Circulation 1977; 56: 786-94.
[32] Reimer KA, Jennings RB. The ‘wavefront
phenomenon’ of myocardial ischaemic cell death II.
Transmural progression of necrosis within the framework of
ischaemic bed size (myocardium at risk) and collateral flow. Lab
Invest 1979; 40: 633-44.
[33] Braunwald E, Kloner RA. Myocardial reperfusion: a
double-edged sword? J Clin Invest 1985; 76: 1713-19.
[34] Chang C, Varghese PJ, Downey J, Bloom S. Magnesium
deficiency and myocardial infarct size in the dog. J Am Coll
Cardiol 1985; 5: 2622-26.
[35] Barros LFM, Chagas ACP, da Luz PL, Pileggi F. Magnesium
treatment of acute myocardial infarction: effects on necrosis in
an occlusion/reperfusion dog model. Int J Cardiol 1995; 48:
3-9.
[36] Christiansen CW, Rieder MA, Silverstein EL, Gencheff NE.
Magnesium sulfate reduces myocardial infarction size when
administered before but not after coronary reperfusion in a
canine model. Circulation 1995; 92: 2617-21.
[37] Herzog WR, Schlossberg ML, MacMurdy KS, et al.
Timing of magnesium therapy affects experimental infarct size.
Circulation 1995; 92: 2622-26.
[38] Leor J, Kloner RA. An experimental model examining the
role of magnesium in the therapy of acute myocardial infarction.
Am J Cardiol 1995; 75: 1292-93.
[39] Thögersen AM, Johnson O, Wester PO. Effects of
intravenous magnesium sulphate in suspected acute myocardial
infarction on acute arrhythmias and long-term outcome. Int J
Cardiol 1995; 49: 143-51.
[40] Barghava B, Chandra S, Agarwal VV, Kaul U, Vashishth S,
Wasir HS. Adjunctive magnesium infusion therapy in acute
myocardial infarction. Int J Cardiol 1993; 39: 13-22.
[41] Schecter M, Hod H. Magnesium therapy in aged patients
with acute myocardial infarction. Mag Bull 1991; 13: 7-9.
[42] Antman EM. Magnesium in acute myocardial infarction:
Overview of available evidence. Am Ht J 1996; 132: 487-95.
[43] Fisher J, Abrams J. Life-threatening ventricular
tachyarrhythmias in delirium tremens. Arch Intern Med 1977; 137:
1238-43.
[44] Levine SR, Crowley TJ, Hai HA. hypomagnesemia and
ventricular tachycardia: a complication of ulcerative colitis and
parenteral hyperalimentation in non-digitalised, non-cardiac
patients. Chest 1982; 81: 244-47.
[45] Antman EM, Lau J, Berkey C, McIntosh M, Chalmers TC,
Mosteller F. Large versus small trials of magnesium for acute
myocardial infarction: big numbers do not tell the whole story.
Circulation 1994; 90(supp I):325.
[46] Shechter M, Hod H, Kaplinsky E, Chouraqui P, Rabinowitz
B. Magnesium therapy in acute myocardial infarction when patients
are not candidates for thrombolytic therapy. Am J Cardiol 1995;
75: 321-3.
[47] Seelig MS, Elin RJ. Reexamination of magnesium infusions
in myocardial infarction. Am J Cardiol 1995; 76: 172-3.
[48] Baxter GF, Sumeray MS, Walker JM. Infarct size and
magnesium: insights into LIMIT-2 and ISIS-4 from experimental
studies. Lancet 1996; 348: 1424-26.
[49] Downing D. The Avoidance of Tabloid Science (Editorial).
J Nutr Med 1991; 2: 347-50.
1359-0847/99/010005-09 $9.50 ©1999Carfax Publishing
Ltd
This page was first uploaded to The Magnesium Web Site on
March 22, 2001
http://www.mgwater.com/


