Magnesium and Public Health: The Impact of Drinking
Water
M.R.H. Löwik, E.H. Groot and W.T. Binnerts
Departments of Animal Physiology and Nutrition
Agricultural University
Wageningen, The Netherlands
Annu. Symp Proc. Univ. Missouri. Trace substances in
environmental health. 16: 189-195 (1982)
ABSTRACT
Magnesium in drinking water has been calculated to constitute
some 10% of the daily population intake. But the effect on public
health must be much larger. This we conclude from results of self
experimentation in a pilot study (M.R.H.L.) with diets of
different Mg levels and a contribution by water Mg. Urinary
analysis revealed that water Mg is absorbed 30% better and much
faster than dietary Mg. There is a considerable diurnal variation
in the amount of Mg absorption, leading to a 50-70% lower Mg
status during the morning hours. When little or no breakfast is
taken, the low Mg period is extended until the coffee break, and
the Mg content of water will then have a crucial importance. The
effect of omitting a meal can be observed during the weekend; it
results in a depression to 70% or less of the usual urinary
excretion. Magnesium status is induced favorably by sports and
exercise, since the necessary high energy diets usually contain
much Mg. In statistical calculations on cardiovascular mortality
due note should be taken of the precipitation of calcium (and
much less so of Mg) during boiling of water. Hence the relative
influence of Mg is larger. Statistics on Mg should be based on
the Mg water content of the working rather than the living
area.
INTRODUCTION
There is a long history of Mg deficiency in cattle, mainly
high-producing milk cows, with frequent occurrence of tetany and
heart disease. The clinical picture is not uniform: sometimes
symptoms of tetany dominate, sometimes there is a labile
situation with muscle paresis, but also sudden heart death may
result. Siollema and co-workers (11,12) were the first
investigators to connect the condition with Mg. It is remarkable
that not all of the cows with extremely low serum Mg are
affected, and the variety of symptoms also suggests a combination
of factors, of which Mg deficiency is a very important one.
In the human the relation of Mg and heart disease is somewhat
different, at least quantitatively. The recommended dietary
allowance is a factor of three larger than the minimum needed to
produce urinary excretion (7). Conformably, serum Mg generally is
in the normal range of .75 to 1.05 mmol/l, whereas in
tetany-prone cows it comes down to one fifth of this value, with
zero urinary excretion. In the so-called "water story" (2,8) the
incidence of human heart disease is inversely related
statistically to the hardness of the drinking water in the living
area. The relationship does not hold in all countries, e.g. in
Britain (5,6) where Mg is generally low, even in hard water. The
question then arises: could it be that Mg is the real driving
force behind the water story? Magnesium has all the required
properties to protect the heart. It ensures the normal quiet
heart rhythm. If this is unduly accelerated by toxic factors or
other stress, Mg may reverse the effect. Magnesium prevents the
so-called "calcium overload". Usually clinicians fight this
condition with pharmaceutical preparations ("calcium blockers")
but Mg would probably do as well, or even better. It also
counteracts digitalis, epinephrine and other toxic agents used to
induce heart disease experimentally in animals. In all of these
conditions Mg restores the original rhythm and effective
functioning of the heart. On the other hand, active Mg is lost
from the ischaemic heart, even before loss of sodium occurs, and
it is replaced later by inactive Mg, probably in the form of
insoluble phosphate. Much of the active Mg is in combination with
ATP, and in the enzyme Na/K-ATPase which stabilizes the Na/K
gradient over the membranes and generates the resting potential
(1,10).
Why is it then that Mg is so often disregarded? As we have
seen, the abnormal human heart condition may occur well above the
deficiency range. Secondly, in the water story, Mg contributes
only little to the daily intake. The contribution has been
estimated as between 1 and 20% (9), or an average 10%, or lower,
in Europe (13). It is difficult to see that such a minor
contribution should have the described important statistical
effect. The aim of this study is to check this low percentage
contribution. It is conceivable that, due to factors like fiber
and phytate, dietary Mg is less efficiently absorbed.
MATERIALS AND METHODS
The research was mainly based on self experimentation. Self
experimentation on one person (M.R.H.L.), while producing only
tentative results, has the advantage of reliability and
flexibility. This was much enhanced by the well established and
fast methods of analysis by atomic absorption spectroscopy. Urine
was collected quantitatively and analyzed in mostly 1:100
dilution. Faeces and food were digested with nitric and
perchloric acid and similarly analyzed. In 4 experimental periods
of 10-14 days duration 3 diets were taken, low, moderate and high
in Mg, whereas in the fourth period the low Mg diet was
reinforced with MgCl2 dissolved in water to the level of the high
Mg diet. The diets were variable, and had only approximately the
calculated content. The exact quantity of Mg taken daily was
determined by duplicate sampling - one tenth scale. Experimental
details will be given elsewhere.
RESULTS AND DISCUSSION
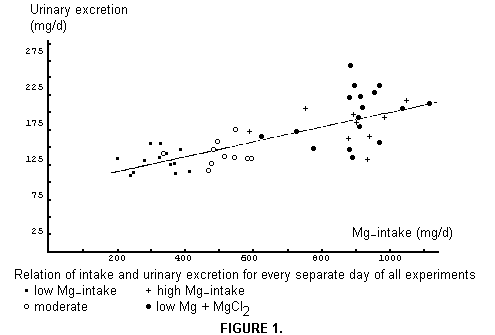
The daily urinary excretion of Mg related to daily intake is
presented in Figure 1. We have added, for this purpose, each
morning's first sample as "night urine" in the total of the
preceding day. It will be noted easily that we did not attempt to
prepare uniform diets, and that most of the daily intakes were
above the 300-350 mg recommendation. Although a line has been
drawn through the point swarm, it only roughly indicates the
relationship, especially because of adaptation effects. As can
readily be seen, the black dots (low Mg + water MgCl2) lie higher
than the black crosses (high Mg diet).
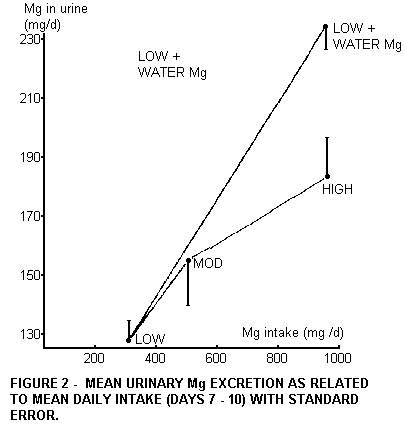
This can be observed more clearly when we omit the preliminary
period, and average the 4 experimental days which start at day 7.
In Figure 2 the averages and the standard error of the mean are
given. Prolonging the experiment by averaging over days 11-14 did
not result in different averages. Our interpretation of the
higher level of urinary Mg excretion with the lowMg + MgCl2 diet
is that Mg is better absorbed from water than from high Mg food.
The difference is 30%.
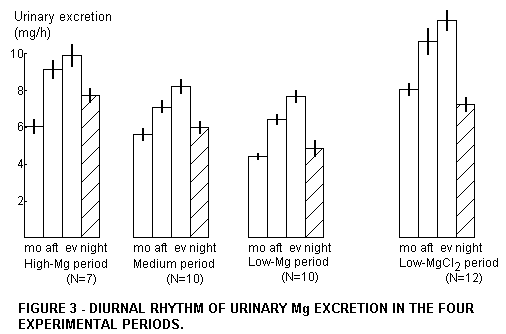
In the beginning of the experiment it was noted that separate
urine voidings of the same day had different Mg contents.
Therefore the experiment was carried out with analysis of the
weighed voidings, usually 4 in 24 hr, and the daily total urinary
excretion was calculated. The subsamples had a diurnal rhythm,
which for the different Mg intakes is presented in Figure 3. We
postulate that these short term effects are only secondarily
generated by variations of the kidney excretion efficiency, and
primarily by variations of the Mg status. (This is confirmed by
Figure 4.) The quantities of Mg excreted per hour generally
increase in the following order: morning, afternoon, evening. The
morning urine then has a value which is a little below that of
the night urine. Only in the experiment with water Mg was the
morning urine higher in Mg than the night urine. This difference
is significant (p<0.05). Obviously Mg from water taken with
breakfast is absorbed much faster than food Mg,
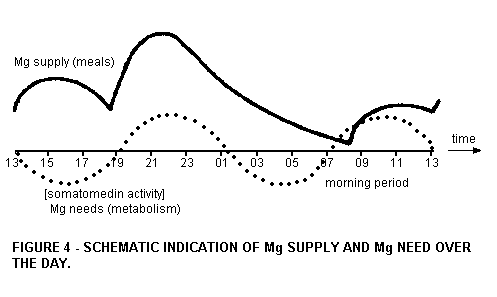
That the morning period has a crucial Mg status is
schematically given in Figure 4. For the construction the
relative Mg contribution of the normal (and low) Mg meals have
been used, as well as literature values on the speed of
absorption and excretion.
During the night hours the Mg status declines considerably, so
that it depends on the following breakfast or morning coffee to
resupply Mg. Magnesium needs, however, are probably high in the
morning hours. We have indicated this with the dashed line in
Figure 4, which we take (3) from the diurnal rhythm of the
hormone somatomedin. Calculations of the levels and the known
relation of somatomedin and the N balance (4) permit computation
of the Mg needs for the morning's resynthesis of cellular
material and plasma proteins. We intend to perform such
calculations elsewhere.
Omitting a meal causes a depression of the daily Mg supply,
easily as much as 30%. Omission of breakfast then has a very
pronounced effect on Monday morning, after the careless weekend.
An example is given in Figure 5, with urinary Mg below 60% of the
mean. Though we did not look for short-term effects, we are
convinced that over short intervals of the morning urinary Mg
excretion may be very low, and when the overall basal Mg status
is low, urinary Mg could be temporarily zero, accompanying a
depressed serum Mg content. The effect of morning coffee,
consumed during such a low Mg period, could be much greater than
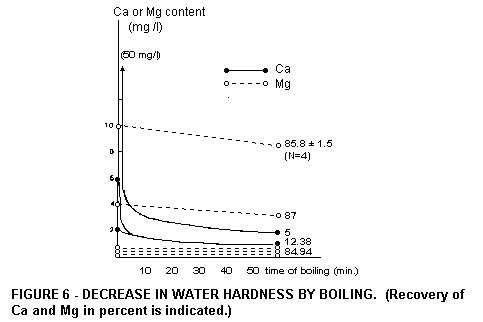
reflected by its Mg contribution to the daily supply. Coffee and
tea are made with boiled water. Hard water, upon boiling, is
rendered soft, but its Mg content is hardly altered (Fig .
6).
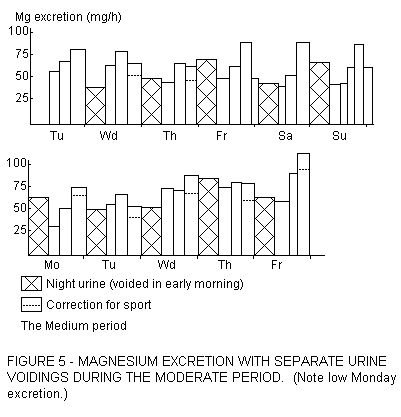
As seen in Fipure 5, sports decrease urinary Mg excretion, and
we have calculated routine corrections for this, amounting to 25%
under our conditions. But at the same time sports, by their need
for energy, increase Mg intake. In modern times no athlete will
choose fat and sugar for his high energy supply, but much more
probably nuts, raisins and chocolate, all with a high to very
high Mg content. Formerly people doing hard work might have taken
beans, peas, etc. ; but also wheat and other grains would have
provided much Mg. Finally we pose the following thesis: benefit
of the training of athletes (and also of heart patients) produced
by exercise is the lowering of the heart frequency by Mg from the
extra food Mg intake. Magnesium, of course, is not the sole
protector of normal heart function, and we do not wish to
over-estimate its effect, but it certainly is one of the factors
that continue to deserve our special attention.
CONCLUSIONS
The short range Mg status of an individual has an appreciable
diurnal variation, with a minimum during the morning hours. This
necessitates hour-to-hour studies instead of the customary
balance studies of several days or weeks duration. Magnesium from
water is absorbed faster and to a higher degree than Mg from
(high-Mg) food. This has an important all-or-none effect on the
morning Mg status, depending on the Mg content of the water.
Although boiling of water softens it, this does not affect the Mg
content, which remains nearly the same. When sport or exercise is
advised to people who are endangered by heart disease, it
involves consumption of high energy foods, which generally
contain much Mg. Part of its effect could be obtained by drinking
water with a high Mg content.
ACKNOWLEDGMENTS
We thank J. Rijken for the pleasant cooperation in the
analytical part. Further we are indebted to experts, especially
C. Kalkman of Amsterdam University, who kindly provided us with
his recent literature studies on Mg and heart/vascular disease,
including the water factor.
LITERATURE CITED
1. Aikawa, J.K. 1981. Magnesium, Its Biological
Significance, CRC Press, Boca Raton, P. 72.
2. Arden, T.V. 1977. Cardiovascular Mortality, An Analysis of
the Water Effect. Fed. Europ. du Traitement de l'eau,
Genéve.
3. Binnerts, W.T., P.W.M. van Adrichem, C.P.J. Oudenaarden,
J.E. Vogt and J.E. Wassenaar. 1982. Plasma somatomedin in dairy
cows: effect of management and feeding level. Neth. Milk
Dairy J. 36:149-152.
4. Binnerts, W.T. and P.M.W. van Adrichem. 1982. Some
observations on plasma somatomedin activity in dairy cows: a
higher correlation with protein than with energy metabolism.
Neth. J. Vet. Sci. (Submitted).
5. Chipperfield, B., J.R. Chipperfield, G. Behr and P. Burton.
1976. Magnesium and potassium content of normal heart muscle in
areas of hard and soft water. Lancet (1) 121-122.
6. Dauncy, M.J. 1972. Urinary excretion of calcium, magnesium,
sodium and potassium in hard and soft water areas.
Lancet (1) 711-774.
7. Marshall, D.H., B.C.E. Nordin and R. Speed. 1976. Calcium,
phosphorus and magnesium requirement. Proc.Nutr. Soc.
35:163-173.
8. Neri, L.C., D. Hewitt and C. B. Schreiber. 1974. Can
epidemiology elucidate the water story? Am. J. Epid.
99:75-88.
9. Neri, L.C. and H.L. Johansen. 1978. Water hardness and
cardiovascular mortality. Ann. N. Y. Acad. Sci.
304:203-219.
10. Seelig, M.S. 1972. Myocardial loss of functional
magnesium. In: Recent Advances in study of Cardiac Structure
and Metabolism I, University Park Press, Baltimore, pp.
615-637.
11. Sjollema, B. 1930. On the nature and therapy of grass
staggers. Vet. Res. 10:425 439, 450-454.
12. Sjollema, B. and L. Seekles. 1930. On disturbance of the
mineral regulatory mechanisms in diseases of cattle.
Biochchem. Z. 229:358-380 (in German).
13. Zoetman, B.C.J. and F.J.J. Brinkmann. 1976. Human intake
of minerals from drinking water in the European Community, In:
Hardness of Drinking Water and Public Health, Pergamon
Press, Oxford, pp. 173-202.
DISCUSSION
Inquirer: M. Van Hardenbrock, Dillon, CO
Q. Correlation of Dutch protein food intake and magnesium
depletion and correlation with hypoxia?
A. This is essentially unknown, and partly so because the
Netherlands Table of Nutrients, 1978, does not list Mg. The level
of protein intake is generally considerable, but some groups are
exposed to "cafeteria type diets", among which are the groups of
"managers". There are Dutch statistics on coronary heart disease
and income (more income, more disease).
Inquirer: John R.J. Sorenson, College of Pharmacy, University
of Arkansas, Little Rock, AR
Q. Did you say that there was Mg deficiency in England?
A. I have said that hard water in England contains remarkably
little Mg, compared to elsewhere.
Inquirer: Herta Spencer, Veterans Administration Hospital,
Hines, IL
Q. There may possibly be an adverse relationship between
protein and magnesium, i.e. that the magnesium requirement may be
higher due to a higher protein metabolism. However, practically
all high protein foods are high in Mg.
A. Thank you for your comment. I have calculated Mg over
protein (in mg/g) and this results in: meat 0.6; fish 2; dairy
products 3 and cereals, nuts, beans, green vegetables and grass
15. (The latter with probably a considerable reduction of uptake
by phytates.)
Inquirer: Carl Marienfeld, University of Missouri, Columbia,
MO
Q. Could it not be an excess of manganese which is the
factor?
A. Statistically, Mg is only one of the protective factors,
with the unique advantage that it has a causal relationship to
ischaemic heart disease. Other trace elements, like Cu and Mn,
are considered to be independent risk factors. But this study
could not be substantiated in at least one recent study (Manthey
et al. 1981. Magnesium and trace metals: Risk factors for
coronary heart disease? Circulation 64:722-729.
This page was first uploaded to The Magnesium Web Site on June
12, 1996
http://www.mgwater.com/






