Nature Medicine, Vol. 1, No. 10, October 1995
The effect of increased salt intake on blood pressure of
chimpanzees
Derek Denton(1), Richard Weisinger(1), Nicholas I. Mundy(2),
E. Jean Wickings(2), Alan Dixson(2), Pierre Moisson(2), Anne
Marie Pingard(2), Robert Shade(3), D. Carey(3), Raymond
Ardaillou(4), Françoise Paillard(4), John Chapman(5),
Joelle Thillet(5) & Jean Baptiste Michel(6)
(1) Howard Florey Institute of experimental Physiology &
Medicine, University of Melbourne, Parkville, 3052 Victoria,
Australia
(2) Centre International de Recherches Médicales de
Franceville, BP 769, Franceville, Gabon
(3) Southwest Foundation for Biomedical Research, W Loop 14,
Military Drive, San Antonio, Texas 78284, USA
(4) INSERM Unité 64, Hôpital Tenon, 75970 Paris
Cedex 20, France
(5) INSERM, Unité 321, Hôpital de la Pitié,
Pavillion Benjamin Delessert, 83 boulevard de l'Hôpital,
75651 Paris Cedex 13, France
(6) INSERM, Physiologie et Pathologie Experimentale Vasculaires,
Unite 367, 17 rue du Fer à Moulin, 75005 Paris,
France
N.I.M. present address: Department of biology, University of
California, La Jolla, California 92093, USA
A.D. present address: Sub-Department of Animal Behaviour,
Madingley, Cambridge University, Cambridge, UK
P.M. and A.M.P. present address: Institut Pasteur De Lyon,
Unité de Parasitologie Experimentale, Domaine du Poirier,
69210 Lentilly, France
Correspondence should be addressed to D.D.
A colony of 26 chimpanzees given a fruit and vegetable
diet of very low Na and high K intake were maintained in
long-standing, socially stable small groups for three years. Half
of them had salt added progressively to their diet during 20
months. This addition of salt within the human dietetic range
caused a highly significant rise in systolic, mean and diastolic
blood pressure. The change reversed completely by six months
after cessation of salt. The effect of salt differed between
chimpanzees, some having a large blood pressure rise and others
small or no rise. These results in the species phylogenetically
closest to humans bear directly on causation of human
hypertension, particularly in relation to migration of
preliterate people, with low Na diet, to a Western urban
lifestyle with increased salt intake. The hedonic liking for salt
and avid ingestion was apt during human prehistory involving
hunter-gatherer-scavenger existence in the interior of continents
with a scarcity of salt, but is maladaptive in urban
technological life with salt cheap and freely
available.
Human societies differ greatly in their food habits, and thus,
in the amount of sodium and potassium ingested. There has been
lively and sometimes acrimonious debate over the role of these
electrolytes, particularly sodium in the genesis of high blood
pressure (1-11). There is evidence from anthropological and
epidemiological studies that high blood pressure, and its
sequelae, cardiovascular morbidity and death, are very largely a
disease of civilization -- that is concurrent with Western
urbanized life style and diet (12-15).
The view of a group of leading researchers in high blood
pressure has been that it is misguided to claim epidemiological
and physiological evidence that the present intake of salt in
Western countries causes high blood pressure (11-16). They hold
that there is an urgent need for properly conducted scientific
studies of the effect of prolonged alterations in salt intake in
order to assess a basis of nutritional advice (11).
Controversy has surrounded the issue of migration of
preliterate people, from characteristically vegetarian 'low blood
pressure societies' with low Na intake to a Western mode of
existence with concomitant diet changes. As well as advent of
stress, or at least stress of a different type, there is weight
gain, and increased Na intake with reduction of K and also often
Ca intake, and sometimes new habits such as alcohol intake and
tobacco smoking. In Kenya such migration from rural society to
Nairobi is accompanied by a fairly rapid increase in blood
pressure over one to two months (17-19). Some authorities
(reviewed in ref. 17) would attribute rise of blood pressure of
such peoples to stress, not to rise of sodium and reduction of
potassium intake shown to occur, specifically citing the contrast
between the certainty of behaviour in a society ruled by ritual
and taboo and the uncertainty of Western societies in which life
is a series of individual choices and decisions (20).
Characteristically, in preliterate societies (12, 21-25) where
diet is largely vegetarian, as in Yanomamo Indians in Brazil
(24,25) and New Guinea highlanders (23), sodium excretion is 1-10
mmol day (-1), potassium excretion is high, 80-200 mmol day (-1),
blood pressure does not rise with age, and there is a very low
incidence of cardiovascular disease. This holds also in the
Bushmen of the Kalahari (22). These people are active and
healthy.
In striking contrast, studies in Japan (26,27) two to four
decades ago showed in rural areas of Northern Honshu that 39% of
the population over the age of 40 had hypertension. Cerebral
haemorrhage was the leading cause of death and was very high
relative to the rest of the world. Mean intake of salt in Akita,
one region so affected, was 450 mmol day (-1) (26 g day
(-1)).
With preliterate low-pressure societies, genetic factors would
not appear to be protective. Like Kenyan tribal farmers, the New
Guinea highlanders upon moving to a Western urbanized environment
with salaried posts at Port Moresby show a clear rise of blood
pressure with age (28).
In an overview (12) it was proposed that a decisive experiment
in this controversial field might be made if salt were added
chronically to the diet of one group of preliterate people, and
not to another otherwise comparable group. This, however, would
not be appropriate because people, once accustomed to the taste
of a diet with high salt content, might not, or could not, easily
give it up.
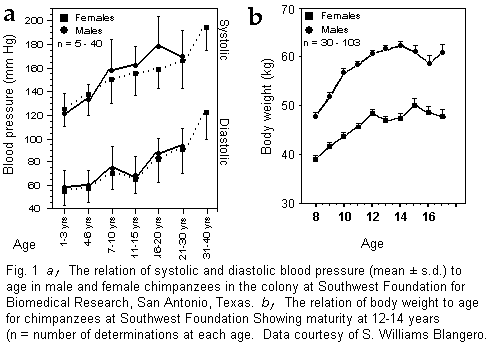
Chimpanzees
Routine veterinary data from Southwest Foundation for
Biomedical Research, San Antonio, Texas, revealed chimpanzees had
an age-related rise of blood pressure (29) (Fig. 1a) analogous to
the population of a Western metropolis. The 1-2 kg day (-1) of
biscuits eaten (Purina Monkey Chow) provided a sodium intake of
about 100-200 mmol day (-1) (6-12 g salt day (-1)); K intake was
high at 140-280 mmol day (-1). J.W. Eichberg and two of us (R.S.
and D.D., unpublished observations) considered trying to reverse
this blood pressure trend by feeding biscuits (Purina) that
contained low salt but were otherwise identical to the Monkey
Chow biscuits (Na content, 8 mmol kg (-1)). The chimpanzees
refused to accept them, and some rapidly lost weight and had to
be restored to the original biscuits, which they readily
accepted.
This gave significance to the opportunity to examine the
influence of added salt on the blood pressure of a colony of
chimpanzees at the Centre International de Recherche
Médicale, in Franceville, Gabon (CIRMF).
The group of 26 chimpanzees, age range 5-18 years, lived on a
vegetarian and fruit diet which gave very low Na and high K
intake. The animals had been living in a number of long-standing,
socially stable, small groups, and it was possible to aggregate
the groups such that there were two groups of 13 that were
matched for age (mean 12.1 and 12.2 years, respectively) and
approximately matched for sex (control, 5 males and 7 females
(one became pregnant and was eliminated), experimental, 7 males,
6 females).
After one year of baseline observations, salt was added in
increasing amounts, 5 g day (-1) for 19 weeks, 10 g day (-1) for
3 weeks, and then 15 g day (-1) for 67 weeks, to the diet of the
experimental group. A six-month foIlow-up period without salt
followed. The essential design of the experiment was that social
conditions of the animals and the vegetarian diet of very high K
content were constant for all animals during the three years. A
liquid infant formula (Cerelac, Nestlé) which provided
liberal Ca as a vehicle for addition of salt was common to both
groups throughout. The single variable that changed was addition
of salt to the diet of the experimental group.
Blood pressure
Blood pressure measurements as a result of the experiment are
recorded in Fig. 2. No significant change in the systolic,
diastolic or mean blood pressure occurred in the control group of
twelve chimpanzees during the two and a half years of the
experiment. Of the 13 animals in the experimental group, 10 of
the animals imbibed all, or a large part, of their allotted salt
ration on most but not all days, consistent with the protocol.
Three others had variable intake sometimes taking in little or
none for several days, and other times taking the full ration
according to protocol. Overall, salt intake was approximately
half that offered. Thus for the first 19 weeks when 5 g day (-1)
was offered, mean intake was 2.6, 2.1 and 3.5 g, respectively,
and when 15 g day (-1) was offered for 68 weeks, mean daily
intake was 7.8, 6.0 and 8.5 g. The results of these three animals
are not included in the statistical analysis and are noted
separately.
With the ten animals (Fig. 2), the salient findings were that
after 19 weeks of salt addition at 5 g day (-1) (total Na intake,
ca. 100 mmol day versus 2-25 mmol day-(-1) in control group) mean
systolic pressure had risen 12 mm Hg relative to baseline
(P< 0.05) with no significant change in diastolic or
mean blood pressure. Following addition of salt at 10 g day (-1)
for three weeks and then 15 g day (-1) for 36 weeks, mean
systolic pressure had risen 26 mm Hg (P< 0.001
relative to mean baseline and P< 0.05 relative to
control group). The mean increases of 5 mm Hg of diastolic and 8
mm Hg mean blood pressure were not significant.
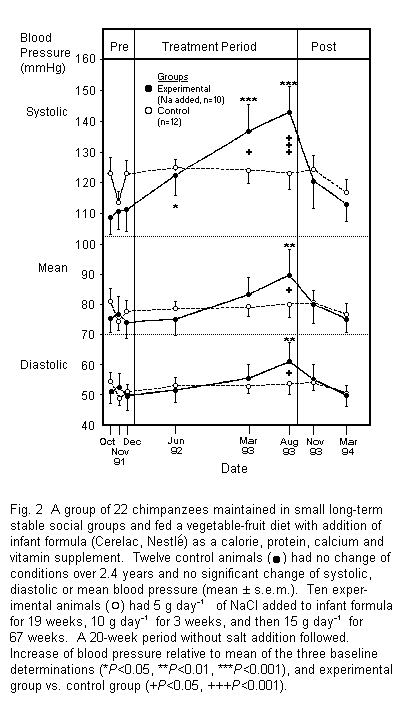
Following a further 26 weeks of addition of 15 g day (-1) of
salt to the food making a total of 84 weeks of added salt, mean
systolic pressure was increased by 33 mm Hg (P< 0.001
relative to both baseline and the control group). The mean
diastolic pressure had risen 10 mm Hg (P< 0.01
relative to baseline and P< 0.05 relative to the
control group). The mean blood pressure was increased by 15 mm Hg
(P< 0.01 relative to baseline and P< 0.05
relative to control group)
By 20 weeks after the end of the period of salt addition, the
systolic, diastolic and mean blood pressures were essentially the
same as two and a half years earlier. Representative individual
records are shown in Fig. 3. Of the three animals that took
approximately half their formula and added salt, two had no
sustained rise of blood pressure, and a third (Fig. 4) increased
approximately 20 mm Hg of systolic, diastolic and mean blood
pressure at the last stage of the experiment when it took more
salt for ca. 25 weeks, an effect that was reversed when salt
intake ceased.

Overall, of the 13 animals in the salt-added group eight (five
males and three females) showed blood pressure increase, whereas
five (two males, three females) did not relative to baseline and
the post salt cessation determinations (Fig. 4). There was no
significant difference in the mean cardiac rate between the two
groups during salt addition to the diet (range, 94-82 beats
min-(-1)).

Body weight
Table 1 records the mean body weight (mean ± s.e.m.)
and mean age of the two groups, and Fig. 1b records the
weights of chimpanzees at different ages at Southwest Foundation
in Texas. Females and males reach maturity at about 12-14 years.
The salient point in the data is that during the first 19 weeks
of the salt addition at 5 g day (-1), the mean weight of the
control group increased 0.3 kg, wheareas that of the experimental
group increased 3.6 kg (P< 0.05). Concomitant with
the addition of salt during the further 65 weeks at a higher
level of 10-15 g day (-1), the increase in weight of the two
groups was similar (experimental 2.3 kg, control 2.1 kg).
Following withdrawal of salt, the mean weight of the experimental
group then declined 2.4 kg over 20 weeks, whereas there was
little change in the control group. The difference in the initial
weights of the two groups results from most males being heavier
in the control group, and the higher final weights of both groups
reflect that the younger chimpanzees grew during the 2.4
years.
Table 1 The body weight of chimpanzees in the control
and experimental groups measured over three years

Blood chemistry
There were no significant differences between the control and
experimental group with respect to plasma [Na], [K], [Ca] or
plasma protein during the experiment. Plasma renin activity (PRA)
was very highly significantly reduced during salt administration:
mean baseline PRA, control, 7.7 ± 1.1; experimental, 6.6
± 1.1 ng AngI per ml per hour (mean ± s.e.m.).
After 19, 58 and 84 weeks of salt intake, the experimental group
values were 3.1 ± 0.9 (P< 0.01), 1.4
±0.8, and 1.4 ± 0.2 (P< 0.001)
respectively and the control values were 8.6 ± 2.3, 5.3
± 1.1 (P< 0.5) and 5.8 ±1.3. Twenty
weeks after cessation of salt, the control group mean was 6.4
± 1.7 and the experimental group was still reduced 3.2
± 0.7 (P< 0.01) relative to the control group.
There was no significant difference between control and
experimental group in concentration of plasma
angiotensin-converting enzyme. With plasma renin substrate, the
baseline control group was 96.9 ± 7.2 versus experimental
89.9 ± 6.3 µ ml (-1) ± (mean s.e.m.). There
was no significant difference between the groups throughout the
experiment except that at the 84th week the control group was
increased (123 ± 26.2 µg ml (-1) relative to the
experimental group 90.9 ± 8.4 µg ml (-1)
(P< 0.01).
Baseline plasma aldosterone (pg ml (-1)) was 95.2 ±
13.2 (control) 111.4 ± 22.1 (experimental) (mean ±
s.e.m.). By 84 weeks the control group values for plasma
aldosterone were 155.2 ± 34.7 slightly
increased(P< 0.05) and the experimental values were
reduced 48.2 ± 9.8(P< 0.05) (control vs.
experimentalp< 0.001). Twenty weeks after cessation
of salt, the control was 132 ± 16.8 and experimental group
126.7 ± 22.1 (not significant). Salt administration caused
a significant rise in percentage of high density lipoprotein
cholesterol (HDL). The baseline levels were control 30.27
± 1.28, experimental group 30.0 ± 2.47. By 58
weeks, experimental mean was 36.78 ± 3.02 (P<
0.001 relative to baseline, and control group, and by 84 weeks
34.60( P< 0.05 relative to baseline and control
group. Twenty weeks after salt cessation, the experimental group
was still elevated 33.37 ± 2.34 (P< 0.05
relative baseline, not significant relative to the control). The
control group was unchanged throughout. The percentage of low
density lipoprotein cholesterol (LDLC) did not change
significantly in either group.
Urine analysis
Clear-cut large differences in Na excretion between the two
groups and the high K excretion in both groups were shown in
24-hour urine collections (Table 2). Creatinine excretion of the
50-kg chimpanzees was consistent with total 24-hour output of
20-25 mg kg (-1) of lean male human and 15-20 mg kg (-1) in the
female (30). Other 24-hour collections of urine showed control
group Na output, 11 mmol day (-1); K, 180 mmol day (-1) (n = 5)
and experimental group Na output, 171 mmol day (-1); K, 164 mmol
day (-1) (n = 7).
Table 2 Twenty-four-hour urinary electrolyte excretion
by chimpanzees in the control and experimental group

* Reflects both animals ate all food but refused half their
salt ration over the 2 days preceding the collections, and
Bernard refused half salt ration on 19/9/93.
There was a very highly significant increase in urinary
Na/creatinine ratio during the salt. The Na concentration of
catheter specimens was 0.9 ± 0.6 mmol 1 (-1) during
baseline, 9.6 ± 2.7 with 5 g day(-1) and 23.6 ± 6.6
at the 84th week with 15 g day (-1)(P< 0.001). The
control group did not change, and there was no significant
difference in the urinary protein/creatinine or Ca/creatinine
ratio between the two groups. Urinary K concentration declined
during salt regime, but K/creatinine ratio was unchanged except
for a significant rise in the mid period of 15 g day (-1) (March
1993).
Discussion
Chimpanzees. These results show unequivocally
for the first time that increased salt intake causes a large rise
of blood pressure in chimpanzees, despite very high K intake
characteristic of a fruit and vegetable diet, and also a liberal
Ca intake (9). Similarly, social conditions of the group were
stable throughout. Thus, in the absence of stress and change of K
status, increase of salt intake to the range of intake in human
societies, 100-200-280 mmol day (-1) (refs 8, 12, 31) (but not as
high as in Northern Japan with people of comparable weight
(26,27)) was the sole cause of a large rise of blood pressure in
the species phylogenetically closest to humans. The blood
pressure reached after 84 weeks was close to that of animals of a
similar age fed a standard high-salt monkey diet over many years
in Texas (29). By 20 weeks after salt ceased, the pressure was
essentially the same as that measured two and a half years
earlier before the salt increase. As seen in humans who have
variable sensitivity to salt (32), eight animals had a large rise
of blood pressure, which reversed when salt addition ceased,
whereas with five there was a small or no change in blood
pressure during salt addition, relative to the levels determined
in baseline period and the last post-salt period for these
animals. In this regard, in Akita, Japan, where mean salt intake
was high (450 mmol day (-1) or 26 g), 39% of persons (with an
average age 45 years) were hypertensive, a larger percentage than
in other communities, but clearly the majority were not. This
fact does not exonerate salt as the major cause of the extant
hypertension, although perhaps this finding is conducive to some
opinions along these lines. These data suggest the value of
analysing this chimpanzee population and other chimpanzee
populations that have variable incidence of high blood pressure
with long-standing consumption of high salt monkey diet,
regarding factors relevant to natriuresis including plasma
ouabain and atrial natriuretic peptide, urinary nitrates, and
also for candidate genes relevant to sodium metabolism. Recent
work has shown that mutation of the β-subunit of a renal
sodium channel is determinant of a type of human hypertension,
Liddle's syndrome (33). The question has been raised whether
variants of renal sodium channel genes affecting function could
be a factor in high blood pressure by causing subtle changes in
sodium handling (33,34).
Migrating preliterate societies. The first
change in blood pressure of the experimental group was a rise of
systolic pressure. Contemporaneously, there was an increase of
3.6 kg of weight compared with 0.3 kg by the controls. This, very
likely, was attributable to fluid retention. A human parallel of
this investigation described cross-sectional studies in Kenya on
2,500 members of the Luo, a tribal, largely vegetarian,
subsistence farming community, which had no cases of hypertension
(17-19). They found that 310 members of the same community who
had originated there and migrated to Nairobi had, by a mean of 41
days later, significantly higher systolic and diastolic blood
pressures, higher urinary Na and lower urinary K output. Weight
increased significantly in males from 57.4 to 60.1 kg. The weight
increase in the migrants was not in keeping with dietary data in
the longitudinal study. There was no evidence of significant
increase of caloric intake. The authors suggested fluid
retention.
Whereas the evidence here shows salt to have a major effect on
blood pressure, this is not to say that in the human situation of
either the migrating unaccultured young adult or with longterm
exposure to elevated salt intake in a Western community, any
increase of blood pressure may not result from a number of
factors, of which salt is one. Salt may be a primary cause but
also possibly permissive of the influence of other factors. It is
a provocative fact that the Yanomamo, "the fierce people" as
characterized in the title of the book by the anthropologist
Napoleon Chagnon (35), have an extremely low Na intake and no
rise in blood pressure with age. Yet, contrary to the view (20)
of a life rendered unstressful by virtue of ritual and taboo,
Chagnon calls their life 'the politics of brinkmanship',
exercising options involving outcomes varying from humiliation,
loss of property and women, severe bodily injury and death.
The role of salt intake in hypertension may reside in the fact
that intake must be above a threshold level of ca. 50-75 mmol day
(-1) (refs 5, 12, 36). As well as a direct causal influence in
those genetically susceptible as, perhaps, exemplified by people
in Akita Province, Japan, ingesting 26 g day (-1), salt, when
above threshold, also becomes permissive for other factors to
operate such as stress, reduced K intake, alcohol or obesity.
Again, this may reflect variable genetic vulnerability. Below
this level there is the characteristic picture of the
unacculturated society, with no increase in blood pressure with
age or incidence of hypertension, this being the natural
physiological circumstance of Homo sapiens.
The increase in the percentage of high density lipoprotein
cholesterol (%HDL) associated with salt was of interest in the
light of the low incidence of myocardial heart disease and high
incidence of cerebral haemorrhage in Japan several decades ago
when salt intake in some regions was very high (26,27).
Salt and human prehistory. Folkow has
expressed views (40) regarding anthropology and the physiological
set point of sodium appetite in animals, inter alia,
suggesting that an elective intake in humans of 150-250 mmol day
(-1) reflects a physiological set point within the hygienic
safety range rather than "hedonistic abuse". He suggests the
studies on preliterate societies represent enclaves not
representative of mankind in general. The statements do not
address the question of why blood pressure does not rise with age
in healthy preliterate societies, including the fact of the New
Guinea highlanders having been shown to excel Royal Australian
Airforce personnel and equal British Commonwealth divisions in
Korea on the physical criterion of the Harvard Pack Test
(23).
During human prehistory the predominant condition has been as
hunter-gatherer in savannah conditions in the interior of
continents (38-40). In the absence of geological sources there is
a paucity of sodium, because 150-200 km from the coast, rainwater
is almost devoid of sodium (12). The major African sites of
prehistoric habitation are in the interior of the continent, as
are the majority of Neanderthal sites in Europe and Asia (40).
The mode of life of contemporary hunter gatherers, the Bushmen of
the Kalahari, the Australian aborigines, the rural Kenyans, and
other preliterate societies living in equatorial conditions is
likely an extant legacy of the prehistory of humanity with a
paramount fact of vegetarian diet (41-42), and constraint on
access to sodium.
It is probably no phylogenetic accident that human breast milk
is very low in sodium (5-9 mmol l (-1) relative to other
mammalian species. This is true also of chimpanzees and gorillas
(43). The hormonal secretary and excretory systems of infants are
being set or honed, to a level compatible with the ecological and
dietetic conditions under which adult Homo habilis,
erectus, and sapiens have lived over the two
million or more years of the late Pliocene-Pleistocene. Also,
with breast feeding continuing for 2-3 years, the mothers could
have a major exposure to Na deficiency, if breast milk (for
example, 1 litre per day) had a higher Na content.
Similarly, the existence of salt-specific nerve fibres and the
propensity to ingest salt when it can get it because the creature
likes it, reflects natural selection of behaviour favourable to
survival under conditions of paucity of supply in which the
species evolved.
With food intake in hunter-gatherer societies, the clear
propensity is to gorge when food is available (44-46). The eating
pattern that has been characterized in studies of the Australian
aborigine as feast or famine can become continuous feast
following exposure to abundant energy-dense foods of Western diet
(45). The sequelae are obesity, insulin-resistant diabetes and
cardiovascular disease in epidemic proportions in the worst
affected communities (46). The Paleolithic diet has been proposed
to represent the nutrition for which human beings are genetically
programmed (47).
In parallel to this situation with food, the hedonic set of
salt appetite in humans and most mammals reflects the
evolutionary history of paucity of access in the face of the
biological imperative of sodium for reproduction. Accordingly, an
avid appetite that was apt when episodic opportunity existed,
akin to food gorging by hunter gathers, is maladaptive today
since technology and commerce has made salt cheap and easily
available, including involuntary intake due to food
processing.
Short of this experiment being done on two groups within a
preliterate human society (12), it is possibly as close to a
decisive experiment as feasible on the influence of salt alone on
a young adult hominid population. It seems apt to suggest that
these data merit close consideration as far as human health is
concerned, particularly in the light of the major population
studies (for example, MRFIT, Multiple Risk Factor Intervention
Trial, involving 350,000 men in 18 US cities) showing continuous,
graded and strong effect of small increase of diastolic and
systolic blood pressure (3-10 mm Hg in both systolic and
diastolic blood pressure above the optimum range of <120
systolic and <80 diastolic) on morbidity and mortality from
cardiovascular disease (48,49), which is the major cause of death
in Western society.
The crucial biomedical consideration may be the influence salt
has in infancy (50), childhood and youth (51) -- the anabolic
phase of life when physiological set points are being
determined-- rather than the relation of salt intake to blood
pressure over some small window of time represented by 24-hour
urine collections in the adult (48), as in the Intersalt
study.
Chimpanzee health. An important outcome of
this experiment concerns the proper diet for conservation and
care of chimpanzee colonies in zoological parks and medical
research institutes. Clearly some nutritional guidelines (52)
embody much too high a sodium chloride content of diet for
welfare of the animals, notwithstanding a high K content. The
Texas data cited (29) on chimpanzee blood pressure also indicate
this. The aim should be a natural fruit and vegetable diet but if
too expensive the biscuit and monkey prepared diet to which the
animals are habituated early in life should not involve Na intake
above 30-40 mmol of Na per day.
Methods
Animals and diet. The large majority of the
animals had been wild caught when very young, mainly as orphans,
and had been at CIRMF for 5-12 years before commencement of
experiment in 1991. Others were born there.
The chimpanzees lived in compatible groups two to six to a
cage, single sex or mixed. The animals of a particular social
group were either all in the control or the experimental group.
The overall health of the animals was excellent throughout the 3
years.
The chimpanzees were fed daily at 0800 h and 1500 h on
bananas, pineapples, tomatoes, cucumber, lettuce and cabbage.
Seasonally, aubergines, peppers, courgettes, avocados, oranges,
mangos, aframomum and passion fruits were added. It was estimated
from analytical food tables (53) and direct analysis that a 50-kg
animal eating 3-4 kg of food day (-1) containing ca. 2000-2500
kcal had a mineral intake of up to 6 mmol Na, 235 mmol K, 12 mmol
per day Ca. Neither Na or K were detected in the colony water
supply.
In 1991, as during earlier years, the animals received 128 g
of a low-Na infant formula (Cerelac, Nestlé) each day
providing a calcium, protein and vitamin supplement. After the
period of control observations (March 1991-January 1992) the
amount of formula offered both groups each day was doubled to
give added volume for the addition of salt to one group (257 g
day (-1) divided into two feedings each dissolved in 750 ml of
water). This was continued until the end of the experiment in
March 1994 (26 months). On a daily basis it provided each animal
with inter alia 1,076 cal, 17 mmol Na, 33 mmol K, and 26
mmol Ca (1.05 g), and added vitamins. With the experimental
group, 5 g day (-1) of salt was added to the formula for the
first 19 weeks, then 10 g day (-1) for 3 weeks, and then 15 g day
(-1) for 67 weeks. The sodium intake of the experimental group
was up to a maximum of 280 mmol day (-1) (see Table 2). One young
animal of the experimental group (Claire, 5 years old (20 kg)
matched with Mebale (5 years) of the control group) was given
half this amount in the same sequence. On each occasion, the
animal's keeper recorded volume drunk.
Twenty-four-hour urine collections were made in an annex to
the cage, which had a collection tray with fine mesh filter.
After 1-2 days of habituation the collections were made over
48-72 h (which reduced the variation arising from non-emptying of
bladder at the end of a period). Infant formula and food regime
were continued in standard fashion. Other 24-hour urine
collections were made on 12 animals in the same conditions. The
area around the annex was monitored to confirm no urine loss had
occurred. Analyses included creatinine determination.
Occasionally, individual animals (such as Makoukou) increased
volume in the urine containers by playing with the drinking
faucet, which provided electrolyte-free water.
Blood pressure and collection of blood and
urine. The animal was anaesthetized in its cage by
administration of intramuscular injection of ketamine by dart
syringe delivered by blowpipe (10-15 mg kg (-1), Imelgene 1000).
The animal was placed on a surgical table in the clinical room
within 5-10 minutes. A cuff of size appropriate for the arm was
chosen (constant for each animal over the 3 years), and the
Critikon Dynamap (5X/5XP Model 1 846) automated record of
systolic, diastolic and mean blood pressure and cardiac rate was
measured from the right arm every minute from thereon.
Twenty-five min after the ketamine, the animal, which was
customarily lying quietly with normal respiration, was given
diazepam intravenously (0.2 mg kg (-1)). The 8-minute interval
between 7 to 15 minutes after the diazepam was the standardized
period of the eight recordings of blood pressure. The mean and SD
of the eight recordings are shown on the graphs of individual
animals (Figs 3 and 4). Any individual reading or readings were
discarded if small movement or arousal occurred during the
standard recording period. Experiments at Southwest Foundation in
Texas on seven male baboons with indwelling arterial cannula
confirmed when following this protocol that blood pressure in the
interval 7-15 min following diazepam administration was not
different from that in the conscious undisturbed state anteceding
intervention (Fig. 5). Following the blood pressure recording,
the chimpanzee had 60 ml of blood collected into Vacutubes, and
urine was collected aseptically by polyvinyl catheter. In the
case of females, cervical inspection ratified that the
intrauterine device used with all mature animals was in situ.
Plasma and urine were refrigerated at -80 degrees C until
transported in polystyrene foam containers packed in dry ice to
Paris for analysis. If animals showed any movement, or evidence
of awakening at any stage, further injections of 1 ml (100 mg) of
ketamine were given intravenously. The animals began to awake 30
minutes after the diazepam injection and were returned to their
home cage. In no instance over the three years, were any medical
or general health sequelae seen to follow the observation
process.

Chemical methods and statistics. Plasma renin
activity and angiotensinogen assay (54), angiotensin-converting
enzyme (55), protein (56), aidosterone (57) and total cholesterol
(58).
A two-way analysis of variance (repeated measures on one
variable) and subsequent t-test (using the error mean square from
the ANOVA as the measure of variability; degrees of freedom, d.f.
of the error mean square, two-tailed test), was used to compare
the values obtained during the treatment or post-treatment
periods to that obtained during the baseline period (for example,
the average of values obtained before treatment with blood
pressure) and to compare values obtained from the experimental
group with corresponding values obtained from the control
group.
The protocol and procedures in the experiment were approved by
the Animal Ethics Committee of CIRMF, and the International
Advisory Committee of CIRMF.
Acknowledgements
This work was supported by The Centre International de
Recherches Médicales de Franceville, the Gabonese
Government and Elf Gabon and the Cooperation Française,
The Howard Florey Biomedical Foundation (U.S.), the Robert J. Jr.
and Helen C. Kleberg Foundation, the INSERM of France, the
Southwest Foundation for Biomedical Research, San Antonio, Texas,
and the National Health and Medical Research council of
Australia. The technical assistance of Irène Laboulandine
is gratefully acknowledged, and that of the animal care staff of
the Primate Laboratory, CIRMF. The generous help in review of
data and manuscript by Edgar Haber, Director, Centre for the
Prevention of Cardiovascular Disease, Harvard School of public
Health, is gratefully acknowledged, as is the help in initiating
the experiment of the late Georges Roelants, Director General of
CIRM in 1991.
RECEIVED 16 JUNE; ACCEPTED 24 AUGUST 1995
1. MacGregor, G. Sodium is more important than calcium in
essential hypertension. Hypertension 7,
628-640 (1985).
2. Dahl, L.K. Salt and hypertension. Am. J.Clin.
Nutr. 25, 231-244 (1972).
3. Simpson, F.O. Salt and hypertension: A skeptical review of
the evidence. Clin. Sci. 57, 463S-480S
(1979).
4. Altschul, A.M. & Groment, J.K. Sodium intake and sodium
sensitivity. Nutr. Rev. 38, 393-402
(1980).
5. Freis, E.D. Salt, volume and the prevention of
hypertension. Circulation 53, 589-595
(1976).
6. Swales, J. Dietary salt and hypertension. Lancet
i, 1117-1179 (1980).
7. Swales, J.D. Salt saga continued. Salt has only small
importance in hypertension [Editorial]. Br. Med. J.
297, 307-308 (1988).
8. Rose, G. & Stamler, J. The INTERSALT study: Background,
methods and main results. J. hum. Hypertens.
3, 283-288 (1989).
9. McCarron, D.A., Morris, C.D., Henry, Hj. & Stanton,
J.L. Blood pressure and nutrient intake in the United States.
Science 224, 1392-1398 (1994).
10. Hanneman, R.L. The politics of sodium restriction in the
United States. 7th Symposium on Salt II, 231-239
(Elsevier Science, Amsterdam, 1993).
11. Brown, J.J. et al. Salt and hypertension. Lancet
ii, 456 (1984)
12. Denton, D. The Hunger for Salt: An Anthropological,
Physiological and Medical Analysis (Springer, Berlin,
1982).
13. Denton, D. Hypertension: A malady of civilization? in
Systemic Effects of Antihypertensive Agents (ed. Sambhi,
M.P.) 577-583 (Symposia Specialists, New York, 1976).
14. Gliebermann, L. Blood pressure and dietary salt in human
populations. Ecol. Food Nutr. 2,
143-156 (1973).
15. Trowell, H.C. From normotension to hypertension in Kenyans
and Ugandans. East Africa Med. J. 57,
167-173 (1980).
16. Laragh, J. H. & Pecker, M.S, Dietary sodium and
essential hypertension: Some myths, hopes and truths. Ann.
intern. Med. 98, 735-743 (1983).
17. Poulter, N.R. & Sever, P.S. Low blood pressure
populations and the impact of rural-urban migration. in
Textbook of Hypertension (ed. Swales, J.) 22-36
(Blackwell Scientific, Oxford, 1994).
18. Poulter, N.R., Khaw, K.T., Mugambi, M., Pearl, W.S. &
Sever, P.S. Migration-induced changes in blood pressure: A
controlled longitudinal study. Clin. exp. Pharm.
Physiol. 12, 211-216 (1985).
19. Poulter, N.R. et al. The Kenyan Luo migration study:
Observations on the initiation of a rise in blood pressure.
Br. Med. J. 300, 967-972 (1990).
20. Pickering, G.W. Salt intake and essential hypertension.
Cardiovasc. Rev. Rep. 1, 1-7
(1980).
21. Page, L.B., Damon, A. & Moellering, R.C. Jr.
Antecedents of cardiovascular disease in six Solomon Islands
society. Circulation 49, 1132-1146
(1974).
22. Truswell, A.S., Kennelly, B.M., Hansen, J.D.L. & Lee,
R.B. Blood pressure of !Kong bushmen in Northern Botswana.
Am. Heart. J. 84, 5-12 (1972).
23. Sinnett, P.F. & Whyte, H.M. Epidemiological studies in
a total highland population, Tukisenta, New Guinea.
Cardiovascular disease and relevant clinical,
electrocardiographic, radiological and biochemical findings.
J. chron. Dis. 26, 265-290 (1973).
24. Oliver, W.J., Cohen, E.L. & Neel, J.B. Blood pressure,
sodium intake and sodium related hormones in the Yanamamo: A 'no
salt' culture. Circulation 52, 146-151
(1975).
25. Oliver, W.J., Neel, J.V., Giekin, Rj. & Cohen, E.L.
Hormonal adaptation to the stresses imposed upon sodium balance
by pregnancy and lactation in the Yanamamo Indians, a culture
without salt. Circulation 63, 110-116
(1981).
26. Takahashi, B. An epidemiological approach to the relation
between diet and cardiovascular lesions and arteriosclerotic
heart disease. Tohoku J. ext. Med. 77,
239-250 (1962).
27. Sasaki, N. High blood pressure and the salt intake of the
Japanese. Jpn. Heart J. 3, 313-324
(1962).
28. Maddocks, 1. Blood pressure in Melanesians. Med. J.
Australia 20, 1123-1125 (1967).
29. Eichberg, J.W. & Shade, R.E. "Normal" blood pressure
in chimpanzees. J. Med. Primatol. 16,
317-321 (1987).
30. Rose, B.D. Pathophysiology of Renal Disease 4
(McGraw-Hill, New York, 1981).
31. Yamori, Y. Hypertensive cardiovascular diseases:
Importance of nutrition in pathogenesis and prevention. in
Third International Conference on Nutrition in Cardiovascular
Diseases. Ann. N.Y. Acad. Sci. 676, 92-104
(1993).
32. Kawasaki, T., Delea, C.S., Bartter, F.C. & Smith, H.
The effect of high sodium and low sodium intakes on blood
pressure and other related variables in human subjects with
idiopathic hypertension. Am. J. Med.
64, 193-196 (1978).
33. Shimkets, R.A. et al. Liddle's syndrome: Heritable human
hypertension caused by mutations in the β subunit of the
epithelial sodium channel. Cell 19,
407-414 (1994).
34. Leckie, B. & Norman, R. A direct link to salt.
Curr. Biol. 5, 260-261 (1995).
35. Changnon, N. Yanamamo: The Fierce People (Holt,
Rinehart and Winston, NewYork, 1977).
36. Kaplan, N.M. Clinical Hypertension (4th edn) 70
(Williams and Wilkins, Baltimore, 1984).
37. Folkow, B. Salt and hypertension. News Physiol.
Sci. 5, 220-224 (1990).
38. WoldeGabriel, G. et al. Ecological and temporal
placement of early Pliocene hominids at Aramis, Ethiopia.
Nature 371, 330-333 (1994).
39. Coppens, Y. East side story: The origin of human kind.
Sci. Am. 270, 88-95 (1994).
40. The First Humans. The American Museum of Natural
History, vol. 1. 46, 54, 65 (Harper Collins, San Francisco,
1993).
41. Clark, J.D. The Prehistory of Africa (Praeger,
New York, 1970).
42. Issac, G. The diet of early man. World Archeol.
(Feb), 278 (197 1).
43. Buss, D.H. Mammary glands and lactation. in
Comparative Reproduction of Nonhuman Primates (ed.
Hafez, E.S.E.) 315-333 (Thomas, Springfield, Illinois, 1971).
44. O'Dea, K. Diabetes in Australian aborigines: Impact of the
Western diet and lifestyle. J. intern. Med.
232, 103-117 (1992).
45. O'Dea, K. Traditional diet and food preferences of
Australian aboriginal huntergatherers. Phil. Trans. R. Soc.
London B334, 233-241 (1991).
46. O'Dea, K. Obesity and diabetes in "the land of milk and
honey". Diabetes/Metabol. Rev. 8,
373-388 (1992).
47. Eaton, S.B. & Kenner, M. Paleolithic nutrition: A
consideration of its nature and current implications. New
Engl. J. Med. 312, 283-289 (1985).
48. Stamler, J. Dietary salt and blood pressure. in Third
International Conference on Nutrition in Cardiovascular Diseases
(eds Lee, K.T., Oike, Y. & Kanazawa, T.) Ann. N.Y. Acad.
Sci. 676, 122-156 (1993).
49. Stamler, J. Blood pressure and high blood pressure:
Aspects of risk. Hypertension 18,
95-107 (1991).
50. Hofman, A., Hazebroek, A. & Valkenburg, H. A
randomized trial of sodium intake and blood pressure in newborn
infants. JAMA 250, 370-373 (1983).
51. Goobbee, D.E. Sodium and potassium. in Textbook of
Hypertension (ed. Swales, J.D.) 539-551 (Blackwell, Oxford,
1994).
52. Nutrient Requirements of Laboratory Animals, No. 10,
Nutrient Requirements of the Monkey (Subcommittee on Laboratory
Animal Nutrition 29-45, (Natn. Academy of Sciences, Washington,
DC, 1972).
53. Paul, A. & Southgate, D. In The Composition of
Foods (eds McCance, R.A. & Widdowson, E.) (HM Stationery
Office, London, 1978).
54. Menard, J. & Catt, K.S. Measurement of renin activity,
concentrations and substrate in rat plasma and radioimmunoassay
of angiotensin J. Endocrinology 90,
422-430 (1972).
55. Cushman, D.W. & Cheung, H.S. Spectrophotometric assay
and properties of the angiotension-converting enzyme of rabbit
lung. Biochem. Phamacol. 20, 1637-1648
(1971). 56. Bradford, M.M. A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the
principle of protein-dye binding. Anal. Biochem.
72, 248-254 (1976).
57. Huu, P., Trung, M.T. & Corvol, P. A direct
determination of plasma aldosterone using a specific aldosterone
antibody. Steroids 24, 587-598
(1974).
58. Doucet, C., Huby, T., Chapman, J. & Thillet, J.
Lipoprotein(a) in the chimpanzee: relationship of Apo(a)
phenotype to elevated plasma Lp(a) levels. J. Lipid Res.
35, 263-270 (1994).
This page was first uploaded to The Magnesium Web Site on June
19, 1996
http://www.mgwater.com/







