Uploaded on January 30, 2002
Importance of magnesium for the electrolyte homeostasis -
an overview
Armin Schroll
Deutsches Herzzentrum München, KIinik für Herz-
und Gefaßchirugie, Lothstr. 1 1, D-80335 München,
Germany
Summary: Disorders of electrolyte homeostasis
are known at many diseases and clinical situations. They have
serious consequences for the cell. Mg-deficiency is followed by a
K-deficiency, which cannot be equalized by K alone: a refractory
hypokalemia always needs additional Mg supply for its
restitution. From K, Mg-deficiency a Na/Ca-overload of the cell
with aggravating consequences will follow: impaired activity and
vitality with electric instability. Mg, which is responsible for
development of a Ca-overload is also able to restore electrolyte
homeostasis by sufficient supply competitively. The
pathophysiologic relations for development of a cellular
imbalance and its restitution concern the Na/K-pump, the Ca-pump
and the Na/Ca-exchange. The clinical applications of Mg therefore
are manifold: recovery under diuretic treatment, coronary heart
disease, arrhythmias, perioperative electrolyte therapy,
transcellular shifts, coronary dilatation and so on.
Introduction
Electrolyte homeostasis - what does it mean? As we know, the
electrolytes sodium, potassium, magnesium and calcium with their
corresponding anions: chloride, phosphate and bicarbonate are
represented within and without the cells at very different
concentrations.
For those electrolytes a transcellular concentration
difference exists with varying gradients between the. intra- and
extracellular space - for example, here at the myocardium.
Naturally there is a tendency for concentration differences to an
equilibrium. It is a characteristic sign of life, however, that
the cell shows a continuous effort against this tendency to
maintain those given gradients with an expense of energy in the
form of a dynamic electrolyte homeostasis. From the concentration
gradients an electric voltage of the cell results, which we call
the electric potential and which is calculable according to
Nernst’s equation:
| |
RT |
|
| Nernst: E= |
|
In CE / CI |
| |
nF |
|
| Table 1. Transcellular electrolyte
gradients |
|
| |
|
ECS |
|
|
ICS |
|
|
| Potassium |
|
4 |
.5 |
|
150 |
mM |
| Magnesium |
|
1 |
|
|
3 |
mM |
| Sodium |
|
145 |
|
|
11 |
mM |
| Calcium |
|
2 |
.5 |
|
0 |
.0001 mM |
Electrolyte imbalance
If the maintenance of the electrolyte gradients under
electrolyte homeostasis is disturbed - for whatever reasons -
then it has serious consequences for the cell:
- their activity and vitality is impaired,
- their action- and resting membrane potential is
decreased
- electric instability develops and
- the cellular membrane becomes permeable for ions.
Only within a certain limit and time will this be tolerable
for the cell. Whenever the limits of injury are exceeded and the
injury is irreversible, cellular death will be imminent. Then
necrosis will develop, which later will be repaired by scarred
tissue. Deleterious consequences for the heart than will be an
insufficiency of the heart after a myocardial infarction or a
rupture.
Imbalances of electrolyte homeostasis are known in many
diseases or clinical situations, especially in patients with
coronary heart disease, arrhythmias and angina pectoris, patients
with acute myocardial infarction, with heart valve disease,
hypertension and congestive heart failure, patients, treated with
diuretics or cardiac glycosides, patients after reanimation or
under intensive care. But trauma, polytrauma or surgical
intervention also will impair the electrolyte homeostasis
especially postoperatively. Many factors predisposing to
electrolyte imbalance arise in open heart surgery with
extracorporeal circulation. Patients suffering from burns need
intensive electrolyte therapy. Furthermore, electrolyte
imbalances must be presumed under excessive and long lasting
stress, in latent tetany, cramps in the legs and diabetes. In the
third trimester of pregnancy magnesium deficiency burdens the
electrolyte situation. Chronic misuse of alcohol is another well
known reason. A very wide field is the influence of hormonal
disregulation on electrolyte homeostasis.
Electrolyte imbalances mainly concern the potassium gradient.
High potassium gradients are characteristic for cells with high
metabolic activity. Skeletal muscles and myocardium belong to
these. Consequently, those tissues are affected first of all.
Together with potassium the other predominantly intracellular
cation magnesium is also involved, because potassium and
magnesium deficiency mostly appear together. An isolated
potassium deficiency is possible but it will remain a rarity.
Lasting over a longer time, magnesium deficiency will always be
followed by potassium deficiency secondarily and because
potassium and magnesium are the most important intracellular
cations, potassium and magnesium deficiency first of all is a
deficiency of the cell.
Table 2. Disturbed electrolyte homeostasis known
at:
- Coronary heart disease
- Traumatic and surgical events
- Dysrhythmias
- Postoperative period
- Angina pectoris
- ECC: open heart surgery
- Acute myocardial infarction
- Burns
- Heart valve disease
- Distress
- Hypertension
- Latent tetany
- Congestive heart failure
- Cramp in the leg
- Diuretic therapy
- Diabetes mellitus
- Digitalis therapy
- Pregnancy
- Cardiac arrest
- Hormonal dysregulation
- Intensive care
- Alcoholism
Refractory potassium deficiency
For a long time it was not clear that with magnesium
deficiency potassium will also be affected, because this
combination must seem very paradoxical. Previously when those
connections were not recognized, surprise was expressed, that an
existing potassium deficiency could not be equalized by potassium
substitution. This situation then was called 'a refractory
potassium dose with refractory hypokalemia refractory potassium
deficiency,' but it was dismissed as a curious event. Later on it
was shown that increasing potassium supply aggravated the
hypokalemic situation more and more. Today fortunately it is
known, that increasing potassium supply will stimulate
aldosterone secretion, so that renal excretion will increase
too.
Cellular electrolyte status
It is difficult to recognize electrolyte status according to
serum values. Only about 2 per cent of the total body potassium
(3600 mmol) is situated in the extracellular space and accessible
there to measurement from plasma or serum. The situation for
magnesium is much worse: only about 1 per cent from the total
body magnesium (1000 mmol) is accessible in the extracellular
space. Therefore it is uncertain whether the intracellular space
with its much higher concentrations is provided with sufficient
potassium and magnesium.
If a deficiency develops slowly, from insufficient supply,
increased loss or higher demand, the organism will be able to
maintain the serum concentrations at the same level for a longer
time at the lower normal range. This will occur at the expense of
the remaining electrolyte stores, which are mainly muscle, bone
and liver. In this situation it is difficult to understand that
serum levels, which are still within the normal range, will not
indicate a potassium or magnesium deficit.
Serum magnesium will not usually be measured, because
magnesium is not routinely measured. But in both cases magnesium
deficiency cannot be recognized, unless deficiency symptoms point
to an electrolyte deficiency or imbalance. Such symptoms may be:
convulsions and cramps of the muscles or cardiac arrhythmias.
Calcium overload and calcium antagonism
For a long time it has been known, that under cellular
potassium magnesium deficiency a calcium overload of the cell
will take place.
This alternating behaviour of magnesium to calcium is called
calcium antagonism and relates back to the fundamental research
of Hans Selye in Montreal and his counterpart Albrecht
Fleckenstein in Freiburg.
Another very essential experience in this field was that
cellular calcium overload can be reversed again with sufficient
magnesium competitively. Magnesium therefore is called the
physiologic calcium antagonist according to this mutual relation
between calcium and magnesium.
Concomitantly to the calcium overload the cell additionally
will be swamped by sodium, whereas potassium together with
magnesium leaves the cell. Summarising, a magnesium deficiency is
followed by a cellular potassium deficiency, which again causes a
sodium and calcium over load of the cell. Then the electrolyte
homeostasis between intra- and extracellular space is disturbed
maximally: a total electrolyte imbalance now dominates.
Table 3. Refractory hypokalemia. 75 year old man,
muscle weakness, diarrhoea, irregular heartbeat, diabetes,
congestive heart failure after AMI
|
|
On
admission |
+40 mmol KCl
i.v. |
+30 mmol
MgSO4 i.v. |
|
| Serum-K |
3 |
.60 |
|
4 |
.80 |
|
3 |
.70 mmol/l |
| Serum-Mg |
0 |
.88 |
|
0 |
.87 |
|
1 |
.18 mmol/l |
| |
|
|
|
| Muscle-K |
33 |
.6 |
 |
34 |
.5 |
 |
45 |
.3 mmol/100g dw |
| Muscle-Mg |
3 |
.67 |
 |
3 |
.62 |
 |
4 |
.26 mmol/100g dw |
| Muscle-Na |
37 |
.4 |
 |
37 |
.5 |
 |
13 |
.2 mmol/100g dw |
|
| Daily: 53 mmol K+ |
|
|
|
|
|
|
|
(Whang 1985) |
Refractory hypokalemia in clinical practice
Those changes have been confirmed experimentally and in
clinical practice.1
A very characteristic example may be the case of a 75 year old
patient suffering from congestive heart failure after myocardial
infarction and from diabetes. Additionally he had diarrhoea and
cardiac arrhythmias. He was also treated with diuretics. These
anamnestic data must suggest a potassium-magnesium deficiency for
many reasons. The cellular potassium and magnesium values,
representatively measured at samples from muscle biopsies, are
very deficient. Muscle sodium is very high, although serum
potassium and magnesium are still within the normal range and do
not suggest any deficiency.
For the first time the patient received 40 mmol potassium
chloride intravenously. The serum potassium value did rise as
expected, while serum magnesium remained unchanged. Also muscle
potassium and magnesium did not change according to a biopsy 12 h
later. This is a typical case for a refractory potassium
deficiency. On the following day the patient received 30 mmol
magnesium sulfate intravenously and again 12 h later the muscle
biopsy showed an increase to normal potassium and magnesium
values in the muscle, while the elevated muscle sodium now was
decreased considerably, This example not only shows the close
connections between potassium and magnesium, it also shows very
convincingly the key role of magnesium for successful potassium
substitution, which would have been impossible with potassium
alone. With potassium chloride alone only a cosmetic correction
of serum potassium was obtained, which was without any influence
at the cellular potassium deficit and therefore it was without
any sense. How can we understand this according to modern
pathophysiologic relations?
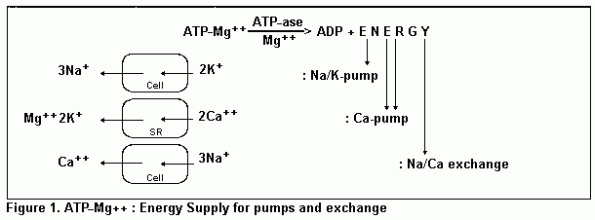
The Na/K-pump
The sodium/potassium pump at the cellular membrane maintains a
high cellular potassium concentration by active transport against
a considerable gradient. The pump is activated by magnesium.
Under magnesium deficiency the pump function is impaired, because
the membrane ATpase, the enzyme responsible, now shows reduced
activity, The energy substrate for the transport activity of the
sodium/potassium pump is represented by ATP in form of its
magnesium complex. This ATP-Mg++ complex is split by
the ATPase delivering the transport energy and therefore it is
said that the ATPase is directing the sodium/potassium pump.
Magnesium deficiency, however means impaired effectivity of
the sodium/potassium pump, whereby insufficient potassium can be
pumped into the cell, although the potassium supply may be great
enough. Therefore the paradoxical seeming statement can be
understood, that magnesium deficiency will be the cause for
potassium deficiency, Furthermore, under magnesium deficiency
there is not enough energy substrate available for the
sodium/potassium pump. The cell membrane now shows increased
permeablilty and the gradients, especially the potassium
gradient, cannot be maintained. Potassium leaves the cell and in
compensation an influx of sodium and hydrogen ions will take
place passively. Also, magnesium leaves the cell, if not enough
ATP is present for forming the ATP-Mg complex and calcium influx
will follow.
The Ca pump and the Na/Ca-exchange
There are two possibilities for elimination of calcium out of
the cell, but unfortunately both are impaired by magnesium
deficiency: this is the calcium pump and the sodium/calcium
exchange. After muscle contraction calcium ions will be
transported back again from the cytosol to the stores of the
sarcoplasmatic reticulum by the calcium pump. The concentration
gradient at this action exceeds several decades and needs an high
expense of energy: one ATP for two calcium ions. The calcium
transport-ATPase there is magnesium dependent, but will be
directed by calcium. The ionic compensation takes place
presumably by one magnesium and two potassium ions.
The other possibility is sodium/calcium exchange. During the
action potential calcium influx takes place along the slow
calcium channels into the cell and induces the contraction
procedure. The calcium influx will be compensated again by an
exchange of three sodium ions into the cell. The energy for the
exchange originates from the high extracellular sodium
concentration, but the three sodium-ions must be removed again
out of the cell by the sodium/potassium pump. For three sodium
ions one ATP is necessary and finally for removing one calcium
out of the cell by sodium/calcium exchange, one ATP is
necessary.3
If the performance of the sodium/potassium pump is impaired,
cellular sodium will increase and inhibit sodium/calcium
exchange. This is due to ATP deficiency, ischemia, myocardial
reperfusion, potassium and magnesium deficiency, hypothermia or
an overdose of cardiac glycosides. In all cases the two impaired
pumps together with the sodium/calcium exchange will lead to an
accumulation of calcium within the cell, called calcium overload.
Magnesium inhibits sodium/calcium exchange only with very high
unphysiological concentrations, but it has an important influence
on it. Increased extra- as well as intracellular magnesium is
able to inhibit competitively the slow calcium-influx through the
calcium channels of the sarcolemmal membrane in the myocardial
cell. But decreased intra- as well as extracellular magnesium
allows an increased calcium influx. This will burden the
sodium/calcium exchange and also the energy demand of the
sodium/potassium pump.
Consequences of electrolyte imbalance
Whenever according to the described mechanisms the electrolyte
homeostasis is disturbed and an electrolyte imbalance of the cell
has taken place, deleterious consequences will follow:
- The decreased resting membrane potential approaches the
fibrillation threshold. The electrical instability of the cell
increases more and more and dysrhythmias will follow.
- At the myocardium the calcium overload induces
hypercontractility with additional useless oxygen and ATP
consumption.
- At the smooth muscles the increased tonicity induces spasms
of coronaries or other vessels with deficient perfusion.
- At the striated muscles a tetanic hyperexcitability
develops with muscular cramps in the legs for example
- and finally the smooth muscles of hollow organs (as bile,
bladder or uterus) incline to cramps, colics and premature
labour.
Under all these conditions of increased energy consumption and
disturbed perfusion, the way directly leads to ischemia of the
concerning cells. Additionally the pump function of the heart is
injured by ectopic beats and tachycardia. Therefore it can be
concluded that magnesium deficiency, the subsequent potassium
deficiency and calcium overload, cardiac ischemia and
arrhythmias, moreover, will intensify themselves, forming a
proceeding multifactorial vicious circle.
Recovery from electrolyte imbalance
The logic consequence to break up this fatal circle of a
general electrolyte imbalance is to transport potassium and
magnesium into the cell:
- for restitution of the transcellular gradients of potassium
and magnesium
- for reactivating of the membrane ATPase by magnesium and
improvement of the sodium/potassium pump and finally
- for reduction of the cellular calcium overload by a
plentiful competitive magnesium supply, with the aim to abolish
the electrolyte imbalance.
The example of the patient shown before could demonstrate,
that the recovery of the electrolyte homeostasis is only possible
with potassium and magnesium together. But it should not take
place under urgent acute conditions, because the reactivation of
the sodium/potassium pump as well as the reduction of the calcium
overload needs some time.
Table 4. Mg-deficiency under diuretic therapy: balance
only after Mg-supply
|
| n=12 |
On
admission |
+40 mmol KCl
i.v. |
+30 mmol
MgSO4 i.v. |
|
| Serum-K |
3 |
.72 |
|
4 |
.33 |
|
3 |
.97 mmol/l |
| Serum-Mg |
0 |
.76 |
|
0 |
.77 |
|
1 |
.35 mmol/l |
| |
|
|
| Muscle-K |
39 |
.5 |
 |
36 |
.0 |
 |
42 |
.3 mmol/100 g dw |
| Muscle-Mg |
3 |
.89 |
 |
3 |
.53 |
 |
4 |
.05 mmol/100 g dw |
| |
| VES/h |
205 |
|
|
178 |
|
|
|
57
|
|
| Dyckner 1979 |
|
|
|
|
|
|
|
|
This is shown by a Swedish study on 12 patients under diuretic
therapy, who had developed a potassium-magnesium deficiency in
spite of regular oral potassium substitution, obviously from the
low values of muscle potassium and magnesium. The serum values
are still within the normal range and do not reflect the actual
existing cellular deficit. But the number of ventricular ectopic
beats, about 200 per hour on average, should point out suspicion
to the disturbed electrolyte situation.4
With supply of 40 mmol potassium chloride intravenously the
serum levels did rise, but the supply was unsuccessuful, because
an elevation of the cellular potassium in the muscle could not be
realized. Only with subsequent or simultaneous additional
magnesium supply after 12 h could normalization of muscle
potassium and magnesium be obtained. It is consistent with the
described pathophysiologic relations, that also the arrhythmias,
induced by diuretics decrease essentially from 205 to 57 per
hour.
Consequences for antiarrhythmic therapy
The logic consequence. however. for any antiarrhythmic therapy
therefore should be to normalize first of all the electrolyte
status as well as the electric potentials at the cell membrane,
before the ionic currents at the cell membrane are influenced by
very effective membrane drugs such as antiarrhythmic drugs.
Possibly several disappointing results of antiarrhythmic therapy
studies in the past are related to the fact that not enough
attention was paid to the electrolyte balance.
Restitution of K, Mg deficiency with Mg alone
Very interestingly the same success as with potassium and
magnesium supply can be obtained with magnesium supply alone.
This case of a 75 year old woman with simultaneous potassium and
magnesium deficiency according to a corresponding Anamnesis
shows, that the cellular restitution of potassium and magnesium
in the muscle is possible by mobilizing the present potassium
stocks, after 30 mmol magnesium was given. The following
potassium supply improves the situation additionally.
Simultaneously the stepwise decrease of the high cellular sodium
in the muscle can be demonstrated in agreement with the recovery
of all the other transcellular gradients.1
All these examples show convincingly that magnesium alone
plays a leading part for development of an electrolyte imbalance
as well as for the recovery of electrolyte homeostasis. With
respect to the fact that the electrolyte balance cannot happen
suddenly, potassium and magnesium should be given as soon as
possible. This will appply to all patients whose anamnesis
suggests a magnesium deficiency or who show typical symptoms of
it. All patients who are treated with diuretics, cardiac
glycosides or cyclosporin need a regular potassium-magnesium
supply.
Table 5. Refractory hypokalemia: 75-year old woman,
increasing weakness, irregular heartbeat, diuretics, congestive
heart failure
|
|
On
admission |
+30 mmol
MgSO4 i.v. |
+40 mmol KCl
i.v. |
|
| Serum-K |
4 |
.10 |
|
4 |
.00 |
|
4 |
.10 mmol/l |
| Serum-Mg |
0 |
.89 |
|
1 |
.43 |
|
|
.99 mmol/l |
| Muscle-K |
38 |
.6 |
|
44 |
.5 |
|
47 |
.4 mmol/100 g dw |
| Muscle-Mg |
3 |
.93 |
|
4 |
.04 |
|
4 |
.43 mmol/100 g dw |
| Muscle-Na |
26 |
.5 |
|
18 |
.8 |
|
13 |
.1 mmol/100 g dw |
|
| daily: 27 mmol K |
|
|
|
|
|
|
|
(Whang 1985) |
Perioperative electrolyte therapy
The electrolyte therapy in patients, who have to undergo
surgery should begin preoperatively, so that an electrolyte
balance without cellular deficits can be presumed at the
beginning of the operation. It is very important to recognize an
electrolyte imbalance in surgical patients and to equalize it,
because many suffer from a potassium-magnesium deficiency as a
result to their previous illness, for example diabetes,
hypertension, coronary heart disease, cardiac valve disease,
increased alcohol consumption or bad condition. The preoperative
recovery of electrolyte homeostasis is very important, because
the subsequent stress and trauma as well as duration or severity
of the operation will induce additional transcellular electrolyte
movements and loss of potassium and magnesium by increased
secretion of catecholamines. The same follows from regional
ischemia, hypoxidosis, acidosis and finally also the activation
of the renin-angiotensin-aldosterone system with increased
aldosterone secretion by renal hypoperfusion. In contrast to
sodium or water, there exists no specific retention mechanism for
potassium or magnesium. The increased renal excretion of
potassium and magnesium by hyperaldosteronism will also continue
if considerable depletion did occur in the organism. This
deleterious fact can be corrected by aldosterone antagonists or
specifically by adequate potassium and magnesium supply.
In the postoperative period it is no less important to
maintain the potassium-magnesium homeostasis, because the patient
in this period is very seriously ill. The catabolic phase, where
potassium and magnesium are liberated from dying cells should
change as soon as possible to the anabolic phase, where much
potassium and magnesium will be necessary for generating new
cells. Therefore also in the postoperative period will be an
increased demand for potassium and magnesium supply.
5,6
Table 6. Surgical electrolyte therapy
Preoperative
- Anamnesis for deficiency and symptoms
- equalizing a deficiency filling up the electrolyte
stores
Intraoperative
- Correcting electrolyte shifts
- substitution of renal losses
- preventing a deficiency
Postoperative
- Substitution according to demand
- catabolism to anabolism
Transcellular shifts under acidosis and alkalosis
For completeness also the changes by electrolyte shifts under
acidosis and alkalosis must be mentioned. Under acidosis hydrogen
ions enter the cell and potassium and free magnesium leave the
cell. The hyperkalemia achieved will stimulate aldosterone
secretion, which will increase renal excretion of potassium and
magnesium. Lactate acidosis under ischemia or ketoacidosis under
diabetes will potentiate cellular potassium magnesium efflux and
aldosterone-induced renal excretion. It is very important to
restore the electrolyte imbalance by potassium and magnesium
supply, if the underlying cause of acidosis cannot be
removed.
Under alkalosis hydrogen ions leave the cell and in
compensation to this potassium and magnesium ions enter into the
cell: hypokalemia will exist. If alkalosis is corrected causally,
a potassium increase will take place, while for the correction of
an acidosis a supply of potassium and magnesium will be necessary
for recovery of electrolyte homeostasis.
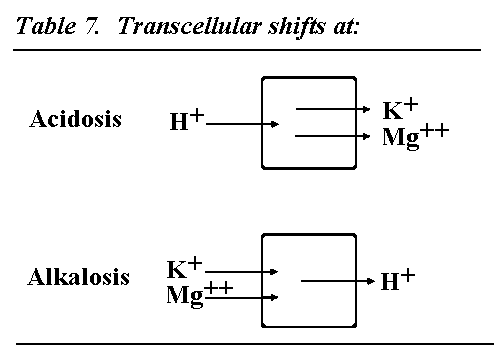
Table 8. Papillary muscle-K and -Mg at mitral valve
replacement with ECC
|
|
|
KCl
(n=10) |
K, Mg
(n=11) |
|
| Pap. muscle-K |
|
|
|
62 |
.4 |
|
83 |
.9 mmol/kg ww |
| Pap. muscle-Mg |
|
|
|
4 |
.9 |
|
9 |
.5 mmol/kg ww |
|
| Von Bormann 1983 |
|
|
|
|
|
|
|
|
K, Mg Substitution is superior to K substitution
From an example shown before, it is evident that muscle cells
take benefit from homeostasis. But it is important to know that
the muscles need much more time for magnesium uptake, than
myocardium or liver. Those organs show a tenfold higher magnesium
uptake, which was shown by the magnesium isotope studies in our
laboratory.
Therefore it was very interesting to examine whether the
results in muscle can also be confirmed myocardium. This
succeeded with direct measurement in the living heart
additionally under maximal surgical conditions of extracorporeal
circulation (ECC) with the highest known electrolyte turnover. In
mitral valve replacement it is possible to take specimens from
papillary muscles, which belong to the support apparatus of the
mitral leaflets.8
Comparing two patient groups with that indication, which were
substituted with potassium chloride on the one side or with
potassium and magnesium together on the other side, this group
shows better potassium and magnesium values in the papillary
muscle.
Thus it is obvious directly in the heart, that
potassium-magnesium substitution is superior also for an optimal
electrolyte balance. According to high transcellular potassium
gradients at the heart thanks to magnesium, the electric
stability of the cell will also be maintained with a high resting
membrane potential and will cause fewer reperfusion- and
postoperative arrhythmias, an experience, which had been reported
from many teams.
Pharmacologically-induced ischemic tolerance
A small magnesium dose, which is not sufficient to normalize
an electrolyte imbalance, can be very helpful according to its
pharmacological calcium-antagonistic effect. In this case here a
unique intravenous dose of only 8 mmol magnesium causes a
transitory deflation of the smooth coronary muscles with a
considerable increase of the ischemic tolerance during coronary
dilatation. The serum magnesium level increases for a short time
over the upper normal range, which is a desirable effect.
Furthermore the ischemia-dependent ST elevation is decreased
considerably and can be recognized primarily after more than
twice the time and with much lower incidence of angina pectoris.
The great advantage is to have more time for the procedure of
coronary dilatation, thanks to magnesium.9
Table 9. Ischemic tolerance at coronary dilatation
(PTCA)
|
|
Mg++
mmol/l |
ST-elevation mV |
after
sec |
Ang.
pect. |
|
| Control |
0 |
.77 |
|
1 |
.32 |
|
19 |
|
+++
|
| + 8 mmol Mg++ i.v. |
1 |
.36 |
|
0 |
.49 |
|
44 |
|
+
|
|
| (Kahles 1991) |
|
|
|
|
|
|
|
|
|
Conclusion
The importance of magnesium for the electrolyte homeostasis
can be summarized now:
- Magnesium deficiency is always followed by a disturbed
electrolyte homeostasis. it causes a lot of deficiency symptoms
according to its many-fold relations in the organism and should
be considered in many diseases and clinical situations.
- Sufficient magnesium supply is important for maintaining
the concentration gradients and the electric potential at the
Cell and stabilizes electrolyte homeostasis.
- Magnesium is able to restore the ionic and electric
imbalance by reactivating the sodium/potassium pump and by
reducing the calcium overload.
- Magnesium is indispensable for potassium substitution and
for compensation of a refractory potassium deficiency.
References
1. Wang, R., Flink, E.B., Dyckner, T. et al. (1985):
Magnesium depletion as a cause of refractory potassium repletion
Arch. Intern. Med. 145, 1686.
2. Hasselbach, W., Makinose, M. (1961): Die Calciumpumpe des
Muskels und ihre Abähngigkeit von der ATP-Spaltung
Biochem. Z. 333, 518.
3. Achenbach, C., Daying, H., Schweikart, P. et al.
(1991): Unterschiedliche Effekte von Mg, Ca, Mn und Nifedipin auf
den Na/Ca-Austausch während des Aktionspotentials der
Herzmuskelzelle. Int. Symposium Edinburgh New approaches in the
Pathophysiology and Treatment of Cardiovascular Disease.
4. Dyckner T., Wester, P.O. (1979): Ventricular extrasystoles
and intracellular electrolytes before and after potassium and
magnesium infusions in patients on diuretic treatment Am.
Heart J. 97, 12.
5. Schroll, A. (1981): Optimierte Magnesium-Substitution bei
extrakorporaler Zirkulation Mg. Bull.
3, 163.
6. Schroll, A. (1986): Magnesium in open heart surgery: its
role in ischemia and arrhythmia Internat. Symposium on
Anaesthesia for Cardiac Patients, München.
7. Wischnik, A., Schroll A., Kollmer, W.E. et al.
(1982): Magnesium-aspartat als Kardioprotektivum und Adjuvans bei
Tokolyse mit Betamimetika Z. Geburtsh. und Perinat.
186, 326.
8. Borman, B. von, Scheld, H.H., Kling, D. et al.
(1983): Concentrations of cations in the tissue of papillary
muscle under different modes of supplementation 5th Annual
Meeting of the Society of Cardiovascular Anesthesiologists, San
Diego.
9. Kahles, H., Riegger, A.J.G., Kromer, E.P. et al.
(1991): Wirkungen von hochdosiertem Magnesium-aspartat wahrend
Koronarangioplastie Deutscher Anästhesiekongreß
Mannheim.
ADVANCES IN MAGNESIUM RESEARCH: 1 MAGNESIUM IN
CARDIOLOGY
Edited by Ronald Smetana
Published as Supplement 1 (1997) to Magnesium Research
ISBN: 0 86196- 555 8
Published by John Libbey & Company Ltd, 13 Smiths Yard,
Summerley Street, London SW18 4HR, England.
Telephone: 0181-947 2777 Fax:
0181-947 2664 E-mail: LIBBEY@Earlsfield.Win-UK.Net.
This page was first uploaded to The Magnesium Web Site on
January 30, 2002
http://www.mgwater.com/


