In Journal of the American College of Nutrition, Vol. 13, No.
5, 429-446 (1994)
Consequences of Magnesium Deficiency on the Enhancement of
Stress Reactions; Preventive and Therapeutic Implications (A
Review)
Mildred S. Seelig, MD, MPH, Master ACN
Department of Nutrition, Schools of Public Health and
Medicine, University of North Carolina, Chapel Hill
The section headers of this paper are as follows:
Stress
intensifies release of catecholamines and corticosteroids, that
increase survival of normal animals when their lives are
threatened. When magnesium (Mg) deficiency exists, stress
paradoxically increases risk of cardiovascular damage including
hypertension, cerebrovascular and coronary constriction and
occlusion, arrhythmias and sudden cardiac death (SCD). In
affluent societies, severe dietary Mg deficiency is uncommon, but
dietary imbalances such as high intakes of fat and/or calcium
(Ca) can intensify Mg inadequacy, especially under conditions of
stress. Adrenergic stimulation of lipolysis can intensify its
deficiency by complexing Mg with liberated fatty acids (FA). A
low Mg/Ca ratio increases release of catecholamines, which lowers
tissue (i.e. myocardial) Mg levels. It also favors excess release
or formation of factors (derived both from FA metabolism and the
endothelium), that are vasoconstrictive and platelet aggregating;
a high Ca/Mg ratio also directly favors blood coagulation, which
is also favored by excess fat and its mobilization during
adrenergic lipolysis. Auto-oxidation of catecholamines yields
free radicals, which explains the enhancement of the protective
effect of Mg by anti-oxidant nutrients against cardiac damage
caused by beta-catecholamines. Thus, stress, whether physical
(i.e. exertion, heat, cold, trauma - accidental or surgical,
burns), or emotional (i.e. pain, anxiety, excitement or
depression) and dyspnea as in asthma increases need for Mg.
Genetic differences in Mg utilization may account for differences
in vulnerability to Mg deficiency and differences in body
responses to stress.
Key Teaching Points:
- Mg deficiency intensifies adverse reactions to stress that
can be lifethreatening.
- Such reactions are mediated by excess release of the stress
hormones: catecholamines and corticosteroids - which are
increased by low Mg and high Ca levels, and which further lower
tissue Mg.
- Low Mg/Ca levels favor excess release or formation of
vasoconstrictive and platelet aggregating factors (derived from
FA metabolism and endothelium).
- Low Mg/Ca levels also directly enhance intravascular blood
coagulation - in microcirculation and in large blood
vessels.
- Eclampsia is a coagulative microangiopathic disorder with
high adrenergic activity; essential hypertension is also a
stress-related disease.
- Low Mg levels are implicated in eclampsia, cardiac
arrhythmias with and without AMI, and bronchial asthma, each of
which is a stressful event that is favorably responsive to
prompt (adjunctive) pharmacologic Mg treatment.
Abbreviations
AMI acute myocardial infarction; Ca calcium; CVD
cardiovascular disease; CS corticosteroid; e.c. extracellular; FA
fatty acids; FFA free fatty acids; GCS glucocorticoid; i.c.
intracellular; IHD ischemic heart disease; i.v. intravenous; K
potassium; MCS mineralcorticoid; Mg magnesium; RDS respiratory
distress syndrome; SCD sudden cardiac death; SIDS sudden infant
death syndrome; PGI2 prostacycline; TXA thromboxane
Stress, both physical and emotional, evokes release of the
stress hormones: catecholamines and corticosteroids, which
mediate release and utilization of substrates for energy
production and for improved skeletal and cardiac muscle
performance. However, their excesses, which cause Mg loss and
inactivation can be implicated in cardiovascular disorders -
involving thrombotic events and arrhythmias, when Mg intakes from
imbalanced diets, and serum and tissue levels are sub-optimal
(Fig. 1).
Fig. 1. Stress and magnesium
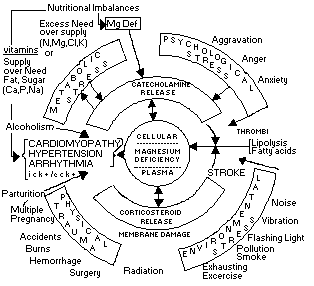
Cardiac complications of stress often derive from the oxygen
debt created by arterial constriction, usually of
arteriosclerotic arteries, that reduces oxygen supply in the face
of (stress-induced) increased energy consumption. There is
suggestive evidence that Mg deficiency contributes to sudden
cardiac death (SCD). Pain of acute myocardial infarction (AMI),
angina pectoris, cancer, trauma, is stressful. In AMI especially
when there is underlying Mg deficiency as is caused by diuretics,
additional Mg loss induced by stress of pain and anxiety, might
be a factor in its morbidity and mortality. Prompt intravenous
(i.v.) pharmacologic treatment with Mg has improved AMI survival.
Mg inadequacy of pregnancy might make additional Mg need during
the stress of labor a factor in periand post-partum problems.
Complications of bronchial asthma and of its drug treatment with
Mg-wasters: beta-adrenergic agonists, corticosteroids (CS), and
theophylline, resemble signs of Mg deficiency that can culminate
in arrhythmia and sudden death. Providing i.v. Mg in doses
comparable to that effective in eclampsia, is valuable adjunctive
therapy in management of intractable bronchial asthma. Mg
supplements have improved endurance and reduced cramps and
fatigue in athletics; might they also protect against SCD of
athletes?
New findings on interactions of prostanoids with Mg provide
insight into how intravascular coagulation is involved in the
pathogenesis of thrombotic arterial lesions that increase
vulnerability to acute changes caused by stress. The mutual
enhancement by the anti-oxidant, vitamin E and by Mg, of their
protective effects against stress-induced myocardial damage, that
is intensified by Mg deficiency, is interrelated with
catecholamine-release of free radicals, as well as with loss of
tissue Mg.
MECHANISMS OF
INTERACTIONS OF STRESS, STRESS HORMONES AND MAGNESIUM
Stimulation by Stress of Secretion of Catecholamines
and Corticosteroids
Classic studies of activation of the sympathetic system by
emotional or sensory stimuli showed that pain, hunger, fear and
rage increased epinephrine urinary excretion (1). During
aggressive and violent action, norepinephrine release
predominates (2). Isolation or overcrowding, forced exercise,
cold or hot environments, noise, light flashes, electric shocks,
or other anxiety-evoking stimuli, including frustration in access
to food, or listening to recordings of fights involving the
species under study - have increased secretion and/or release of
catecholamines by the adrenal medulla, nerves and ganglia. The
heart also synthesizes, stores and releases norepinephrine (2-5);
almost immediately after a coronary occlusion, catecholamines are
released from granules within the heart (5,6).
Elevation of plasma CS and increased urinary excretion of CS
and its metabolites have been reported in monkeys subjected to
anxiety or aggression (7), and in humans under emotional and
physical stresses (2,8-10).
Stress Reactions as Affected by Magnesium and
Calcium
Interrelations among Catecholamines, Magnesium and
Calcium. Catecholamine-secreting granules from adrenal
medulla or nerve endings, suspended in low Mg/high Ca or high
Mg/low Ca solutions, release more catecholamine in low Mg and
less in high Mg media; Ca has reciprocal effects(11-15). (Figure
2) The effect of verapamil, a Ca channel-blocker, on
catecholamine release has been compared to that of Mg, a
physiologic Ca-blocker (16).
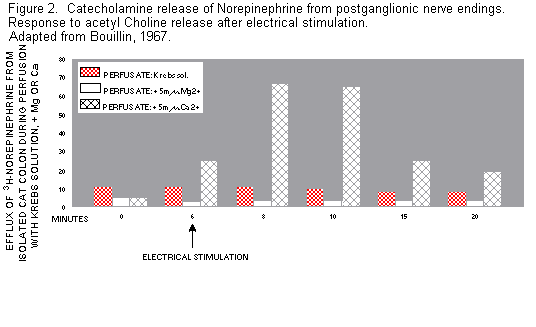
Hypomagnesemia occurs in patients with elevated blood
catecholamines: in AMI, cardiac surgery and insulin-induced
hypoglycemia stress tests (17). Epinephrine infused into healthy
volunteers, with and without prior treatment with Ca-blocking
agents, lowered both serum Mg and K (18). Infusion of
pathophysiologic amounts of epinephrine, or a therapeutic dose of
salbutamol (a beta 2-catecholamine agonist) lowered plasma Mg
levels in normal subjects. Epinephrine, but not norepinephrine,
significantly reduced plasma Mg in healthy men (19). Infused
beta-blockers had no significant effect on plasma Mg in 15
sedentary, healthy young men when maximally exercised (20).
Mg infusion (MgSO4, 60 mg/kg, i.v.) improved management of
patients with pheochromocytoma prior to and during surgery to
remove the catecholamine secreting tumor (21), and inhibited
release of catecholamine produced by the stress of tracheal
intubation (22). Experimental and clinical hyperadrenalemia
caused elevated blood Mg levels, that fell to within normal
limits after extirpation of an adrenal gland (23,24). The high
erythrocyte (rbc) Mg (24) may be to the increased rbc Mg levels
of most athletes, whose release of catecholamines increases
during strenuous training (infra vide).
Corticosteroids during Stress; Interrelations with
Magnesium
Prolonged isolation and other emotional, but not physical,
stress has increased serum CS in rats (25). Runners exhibited
increased excretion of both catecholamines and CS; Mg supplements
significantly decreased their CS excretion (9). A marathon
runner, whose CS levels gradually increased during a race,
attained twice pre-race values at the end of the race (26). A
mixed CS, with glucocorticosteroid (GCS) and
mineralocorticosteroid (MCS) activity, caused negative Mg balance
in normal volunteers, mostly by interfering with intestinal Mg
absorption (27).
MCS hormones: aldosterone and desoxyycortosterone acetate
(DOCA) affect Mg metabolism and are influenced by the Mg status.
Each interferes with Mg absorption and increases urinary Mg
excretion in experimental animals (28,29) and in humans with
adrenal tumor-induced aldosteronism (30,31). Mg deficiency has
increased MCS secretion in experimental animals, through induced
juxtaglomerular hypertrophy (32,33). Anti-aldosterone agents
reverse Mg deficiency of cardiac patients on long-term use of
Mg-wasting diuretics, as reflected by restoration of depressed
tissue Mg levels (34,35).
There is substantial experimental evidence (2,26,36-49) that
cardiac damage - induced by stress or exogenous catecholaminesis
intensified by CS, beta-adrenergic agonists, and by Mg
deficiency. The myocardial lesions are characterized by necrosis
and Ca deposition; Mg administration is protective.
Catecholamines, Fatty Acid Release, Coagulation, and
Prostanoids
Fatty Acid release of Stress; Interrelations with Mg.
Free fatty acids (FFA), an energy source during stress, are
mobilized through lipolysis induced by beta-catecholamines
(50,51). However, they bind and inactivate Mg in blood and heart,
intensifying functional Mg deficiency. The stress of alcohol
withdrawal increased serum FFA and lowered serum Mg (in dogs
[52]). AMI increases catecholamine release and increases FFA
levels (53,54), that is associated with a decline in serum Mg
(55). Hyperexcitable (Type A) subjects, who are more vulnerable
than Type B subjects to AMI, exhibit greater adrenergic release,
have increased serum FFA and slight increase in plasma Mg and a
small but significant decrease in rbc Mg (56).
Among five marathon racers, four were well trained; an
untrained racer had been taking a Mg supplement (370 mg/day) for
a week before the run (57). Blood samples drawn 6 times during
the race showed steady increase in mean FFA that peaked at 26
miles, and that was associated with a steady fall in serum Mg in
the trained runners, but not in the untrained, Mg supplemented
man.
Fat and Magnesium/Calcium Ratios in Coagulation.
Diets rich in saturated fats are implicated in hyperlipidemia and
atherosclerosis, and increase thrombogenesis. Mg deficiency
worsened both fat-induced intravascular hyper- coagulation
(58-61) and atherogenesis; Mg was protective (42,46,59). It has
prevented platelet aggregation on experimentally damaged
endothelium (60,61), and has protected against spontaneous MI of
rats, dogs and cocks on nutritionally imbalanced diets that
caused Mg deficiency (42,62,63). Increased platelet aggregability
increases myocardial vulnerability to ischemic injury in Mg
deficient hamsters (64). The reciprocal effects of Ca and Mg on
coagulation are considered elsewhere, re the need for Mg
supplements of post-menopausal women taking both estrogen and Ca
to slow osteoporosis (65), and in eclampsia, premenstrual
syndrome, and migraine (66).
Platelet Aggregation and the Prostanoids, Affected by Mg
and Ca. Prostacycline (PGI2) and thromboxane (TXA or TXB2)
also participate in platelet aggregation, PGI2 inhibiting
aggregation; TXA enhancing it. PGI2 is also a potent vasodilator;
TXA is a vasoconstrictor. Guenther et al have reported that
increased prostanoid synthesis is linked to translocation of Ca
into cells (67). Mg deficiency (which causes hypercalcemia in
rats) caused increased levels of PGI2 and its metabolite PGF1,
but to a far lesser extent than it increased TXA synthesis and
release. Studies by Nadler et al (68-70) in normal men have shown
that Mg infusion significantly increased excretion of the PGI2
metabolite 6-keto-PGF1a, without altering urinary output of PGI2,
and reduced TXB2 synthesis. Franz et al (26) found that physical
stress of marathon racing increased TXB2 levels, inversely
related to serum Mg levels. Early in the race TXB2 fell slightly,
but it rose 9-fold by the end of the race, in association with a
fall in serum Mg. PGI2 mediates, at least in part, the
hemodynamic (vasodilator) effects of infused Mg (71). Altura et
al (72) showed that, in its absence, PGI2-induced relaxation in
isolated rat aortic strips is prevented. PGI2 and Mg infusions
elicit similar hemodynamic effects (73), and in vitro exposure of
(umbilical) vascular endothelial cells to vasodilating
concentrations of Mg stimulates release of PGI2 (74,75).
Catecholamines, Free Radicals, Magnesium and
Antioxidants. Recent work in the laboratories of Weglicki
and Bloom (76-83) indicates that oxidative stress, induced by the
beta-agonist isoproterenol, causes membrane damage of myocardium,
endothelium, and erythrocytes in which release of free radicals
participates. Mg deficiency and catecholamines each causes tissue
Ca overload. Both beta-blockers and Ca-channel blockers cause
degrees of membrane lipid anti-peroxidative activity (76-81).
Furthermore, catecholamine auto-oxidation leads to generation of
cytotoxic free radicals (84). That the cardiomyopathy of Mg
deficiency, alone, also involves free radicals is indicated by
the protective effects of vitamin E and anti-oxidant drugs in
Mg-deficient hamsters (80-83). This is pertinent to the
observation that high intakes of anti-oxidant nutrients, as well
as of Mg, were cardioprotective in a large series of Indian
cardiac patients (Singh et al [85,86]) .
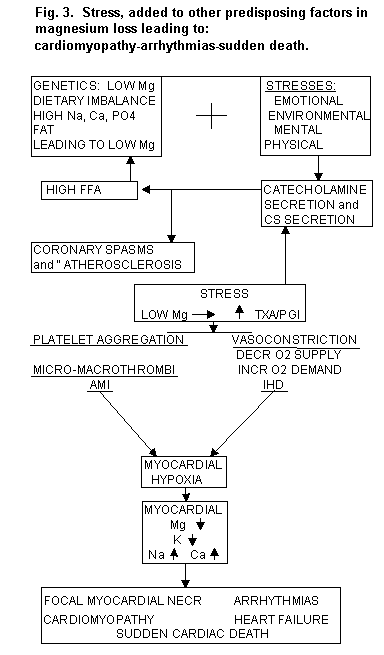
Cardiac Synthesis and Release of Catecholamines;
Cardiac Complications with Excess Catecholamines
Raab first reported very high catecholamine content in the
myocardium of a young athlete who died in his sleep after a
stressful event (3). The inotropic response to increased demands
caused by stress increases the force of cardiac contraction and
oxygen utilization (4). Resulting oxygen debt causes relative
myocardial hypoxia that can contribute to a shift of Mg out of
cells to the extracellular space and plasma, as occurs with local
ischemia of skeletal muscle (87) and in infants with asphyxia
(88). The inward shift of Ca, stimulated by catecholamines is
important in cardiac contractility, but when excessive, as in
patients with ischemic heart disease (IHD) - the chronotropic
responses to catecholamines predominate, and there is increased
risk of arrhythmia and myocardial damage (89). Even in normal
subjects, especially if low in Mg, the chronotropic effect of
stress-induced excessive catecholamines may cause arrhythmia and
sudden cardiac death (SCD). The increase in FFA caused by stress
plays an important role in reducing availability of myocardial
Mg. Focal myocarditis and congestive heart failure (CHF), common
in patients with pheochromocytoma (90), is the clinical
counterpart of the focal myocardial lesions seen in rats given
high dosage norepinephrine (91), and in those caused by injection
of a beta-adrenergic agonist (isoproterenol), at a dose just
sufficient to cause multifocal micronecrosis (40,92). Mg loss is
the earliest electrolyte derangement, preceding loss of K and
gain of Na and Ca.
Functional stress tests, to which IHD patients are subjected,
evoked significantly higher epinephrine and aldosterone levels in
cardiac patients than in healthy controls (93). Pre-stress serum
Mg levels were 1.6 mEq/L, and fell further at the test end.
Influence of Personality on Cardiovascular Responses to
Stress and Magnesium
Emotion/anxiety, as well as ischemia, interfere with
myocardial oxygen economy (2). Emotion evokes outpouring of
catecholamines and CS, which deplete myocardial Mg, as the
central cardio-damaging factor of stress, leading to tachycardia,
arrhythmia, cardiomyopathy and even SCD. Nervous, emotional
individuals who are most prone to cardiovascular disease (Type A)
have far less stress tolerance than do Type B persons (94).
Exposure to noise and mental stress results in excretion of more
catecholamines by Type A than by Type B subjects, who have higher
blood Mg (most marked in erythrocytes) than do Type A subjects
(56,95). Henrotte et al have found that 70% of variance in rbc Mg
levels is familial (56,95,96). Type A students, given a
standardized test, had a statistically greater fall in rbc Mg
than did Type B students, similarly stressed (56). The
self-sustained stress and exaggerated response to external
stresses of Type A persons might lead to subnormal Mg status.
Patients with latent tetany of Mg deficiency, who have
psychoneurotic complaints (97,98), may also be especially
vulnerable to mitral valve prolapse (97,99,100). Children with
"nervous" complaints related to psychosocial and school stresses
are also prone to hypomagnesemia (101). Durlach (97) suggests
that whether Mg deficiency is acute or marginal and chronic (as
with long-term suboptimal Mg intake, genetic Mg malabsorption,
renal-wasting, or maldistribution), it increases vulnerability to
stress, and increases its harmful effects.
Hypertensive Responses to Emotional Stress; Mg/Ca
Effects
Emotional stress is a factor in hypertension (2,102). Whether
clinical hypertension is associated with low or high plasma renin
activity (PRA), free i.c. Mg is low (103-107); there is negative
correlation of PRA with serum Mg levels (103). In low PRA
patients, whose blood pressure was reduced by Ca supplements, and
high PRA patients who respond to Mg, free i.c. Mg was inversely
correlated with degree of hypertension (104). Low free i.c.Mg was
also seen in rats made hypertensive whether by MCS and salt
loading, by nephrectomy or renal ischemia (107). Low i.c. free
rbc Mg was found in thin and obese hypertensive patients, in
hypertensive obese patients with or without diabetes mellitus,
and in diabetics (106).
In a study of total rbc Mg levels (a less sensitive parameter
than the free rbc Mg) of middle-aged patients with labile
hypertension, only those with low total rbc Mg had a blood
pressure-lowering response to three months of Mg supplements
(108). Workers in a high noise environment, and students
preparing for their final examination experienced a rise in blood
pressure during the work or study period (109) on diets providing
about 5 mg/kg/day, which is above the current official American
Recommended Dietary Allowance (RDA) (110). There was no rise in
blood pressure in workers, or in students, given Mg
supplementation that increased daily Mg intake to 6-7 mg/kg/day
(109).
TOXEMIAS OF PREGNANCY,
POSTPARTUM CARDIOMYOPATHY AND SIDS
Magnesium, Platelet Aggregation and Prostanoids of
Toxemias of Pregnancy
Although only indirectly related to acute stress, aside from
labor - which can intensify damage associated with toxemias of
pregnancy, hypertension, that responds to Mg treatment, is
characteristic of pre-eclampsia, as is greater than normal
increase of blood pressure following catecholamine injection
(111). Increased urinary epinephrine has been found in most
patients with convulsive eclampsia (112).
Elucidation of interrelations of Mg on PGI2, TXA and
endothelial-derived factors provides insight into the
hypertension and hypercoagulability of blood of toxemic
pregnancy, and draws parallels to that of cardiovascular disease
and diabetes mellitus (113). The reversal of increased blood
coagulability of women, with toxemias of pregnancy, by infusions
of Mg was demonstrated first by Weaver (114), His pregnant ewes
on Mg-low diets had hypertension, renal glomerular endotheliosis,
and placental infarcts (115,116).
The increased Mg requirements of pregnancy, and the favorable
response of complications of pregnancy to Mg treatment are well
known (59). Mg deficiency during complicated pregnancy might be
contributory to microvascular thromboses of severe toxemias. Pain
from labor, as a stress factor that further increases Mg needs
might be a factor in peripartal cardiomyopathy. It is provocative
that Woods' rationale for extension of trials of Mg in AMI
patients (71) derived partially from its efficacy in eclampsia,
and from studies of Mg/PGI2 relations in pregnancy (74,75).
Vascular endothelial damage (reflected by elevated fibronectin
levels in preeclampsia), is associated with hyper-aggregability
of platelets, and linked with prostanoid disturbances and
microvascular thrombi in more severe toxemias (75,117). Watson et
al (75) found that eclamptic patients have depressed PGI2 levels,
whereas serum from MgSO4-treated eclamptic patients increased
release of PGI2 by cultured (human umbilical vein) endothelial
cells, and that it overcame thrombin induced- platelet adhesion
to endothelium. Calvin et al (118) found that plasma fibronectin
(released from damaged endothelium) was higher in 18 preeclamptic
women than in 19 normal pregnant controls. However, the
metabolite of PGI2 (6-keto-PGF1x: which they suggested is
possibly a poor index of PGI2 at the microvascular level) was
also higher. In vitro studies of the effect of Mg on platelet
aggregation in umbilical cord vessel showed that elevated Mg
levels increased PGI2 release from the vessels, and increased the
anti-aggregating response of platelets to PGI2 (75).
Relationships of Perinatal Mg Deficiency to Infantile
Reactions to Stress
Low birth weight (LBW) infants are more commonly born after
complicated pregnancies, that are associated with Mg loss, and to
Mg deficient mothers than after Mg-replete pregnancies (59). Not
only are such infants more prone to early infantile
complications, they are subject to serious reactions to stress.
Dietary Mg deficiency of ruminants has caused birth of low birth
weight young (115,116). Rats born to Mg deficient dams are less
tolerant of stress, even after infancy (119). Pregnant Mg
deficient cows and ewes exhibited 3-fold more clumping of
platelets than did controls, and six of 18 lambs, that died in
tetany after being fed low Mg diet for 2-12 months, had heart and
lung lesions very similar to those produced by
collagen-activation of platelets (120). Miller et al (120)
suggest that abnormal blood platelet activation may be a
significant mortality risk factor in severe gestational and
infantile Mg deficit, and draw a parallel between the pathologic
findings in Mg deficient lambs with those in the sudden infant
death syndrome (SIDS). Caddell proposes that neonatal and
postneonatal apnea and respiratory distress syndrome (RDS) are
premonitory findings in infants who are later victims of SIDS,
and advises Mg treatment in doses low enough not to constitute a
risk (0.4 -1 mEq/kg/d for about 2 weeks) in RDS infants with
immature kidneys (121-123). Infants with RDS are most commonly
born to mothers subject to hypomagnesemia (59,124). SIDS has
pathogenic features analogous to sudden cardiac death (SCD) of
adults, in which stress and Mg deficiency have been implicated as
factors (59,125-127).
MIGRAINE , COAGULATION
AND PROSTANOIDS
Among the conditioning factors considered in migraine is
stress, with its induced intravascular coagulability, associated
with hypomagnesemia, platelet hyperaggregability and decreased
cerebral blood flow (66,115,128-130). Weaver (115) has correlated
the high incidence of migraine in patients with eclampsia (131)
with the platelet hyperaggregation seen in both conditions (132).
Altura (128) hypothesizes that the Ca-blocking effect of Mg might
justify its trial in migraine, to prevent or ameliorate the
initiation of migraine attacks.
NOISE STRESS ,
HEARING LOSS AND ACCELERATED AGING
Among the stresses that increase vulnerability to cardiac
damage in rats is noise. It increased Mg loss from the heart, and
myocardial Ca and collagen deposition (133). Franz found impaired
hearing of surviving Mg deficient litter-mates, some of whom had
died of sound-induced seizures (134).
Noise stress damaged the inner ear; Mg deficiency intensified
ear damage; high Mg intake was protective (133,135-138). Ising
and Joachim et al have negatively correlated hearing loss with
perilymph and rbc Mg levels (133,138), and suggest that energy
requirements of the inner ear are compromised by Mg
deficiency-induced increased catecholamine secretion and
arteriolar constriction. Mg deficient rats exposed to noise over
a long period (i.e. 30 months), age rapidly (133). Cold stressed
rats, made Mg deficient enough to cause adverse reactions and
death of some in infancy, but to which adaptation took place in
survivors, experienced both reduced tolerance of cold stress (as
reflected by cardiac damage), and shortened life span (139).
OBESITY AND STRESS OF
STARVATION
Voluntary starvation to reduce obesity is not the severe
stress of famine, or of concentration camps - which has been
associated with myofibrillar disruption (140) and SCD (141).
During refeeding of surviving victims, strongly negative Mg
balances persisted for months on Mg intakes as high as 800 mg/day
(142). Even starvation to lose weight has caused arrhythmias and
sudden death, possibly associated with lipolysis of ample fat
stores, which as with catecholamine released during stress,
inactivates available Mg (supra vide). DeLeeuw et al (143),
commenting that sudden deaths of total starvation and some
low-calorie diets are probably cardiac, showed that total
starvation in an obese animal model caused severe myocardial Mg
depletion.
Shortand long-term fasting of normal volunteers has caused Mg
loss, but not necessarily hypomagnesemia (144,145). Balance
studies of grossly obese men who were fasted up to 3 months
showed urinary losses to be responsible for loss of 20% of the
body Mg, that resulted in development of carpal spasms requiring
MgSO4 infusions for relief (146). Although the Mg status was not
explored in the patient whose death after fasting had been
preceded by lactic acidosis (147), it is pertinent that high
lactate increases urinary Mg loss (148). A healthy young woman
developed refractory hypokalemia during starvation for obesity,
taking amino acid and protein supplements, despite high dose K
treatment (149). Her plasma Mg was normal at the time of death
from ventricular fibrillation after elongated QT interval and
persistent arrhythmias (seen with hypomagnesemia or hypocalcemia)
were treated with Ca infusion. Gross myofibrillar destruction was
found, like that described with experimental Mg deficiency (150)
and in starvation victims (140). Comparable myocardial lesions
were seen in a 38 year-old woman who died in ventricular
fibrillation after a liquid protein diet, supplemented with
multivitamins, Ca and K (151); i.v. Mg treatment of her
refractory ventricular tachycardia had not increased her serum Mg
above 1.6 mEq/L. Potentially life-threatening and fatal cardiac
arrhythmias have been reported in patients taking liquid protein
reducing diets (152-154). Most striking, in a study in which the
Mg levels were followed, was persistent magnesuria and normal
serum Mg levels despite low intake (153); three of the subjects
developed arrhythmia (154).
GASTROINTESTINAL STRESS REACTIONS;
HISTAMINE RELEASE
Classen's studies of the alarm reaction are pertinent to
gastrointestinal (GI) reactions to stress (155). Peptic ulcers in
rats are characteristic of the early stress reaction, which
sensitizes the mucosa to irritants and other stimuli, especially
in Mg deficiency (156-157). Their incidence and extent were
decreased by Mg administration. The effect of Mg deficiency on
intestinal motility (157) may help to explain the stomach aches
and acute abdominal pain in nervous children with marginal Mg
deficiency and the beneficial effects of Mg supplementation in
these patients (157,158).
How Mg inhibits stress-induction of peptic ulcers is
uncertain. Several biochemical changes (decrease of ATP, ADP, and
extent of phosphorylation reactions, and increase of cAMP, AMP)
preceded the macroscopic appearance of stress ulcers in normal
stressed rats (159). The Mg status was not explored, but it is
noteworthy that ATP synthesis is Mg-dependent, as is
phosphorylation (160), while cAMP levels are low in Mg deficiency
(161). In view of the classic use of histamine to test for
gastric acid secretion, and the usefulness of histamine
receptor-antagonists in management of peptic ulcer disease, it
seems plausible that Mg deficiency intensifies stress ulcers
through its stimulation of histamine secretion. Mg deficiency in
rats increases degranulation of mast cells with histamine release
(162-164).
Bronchial asthma (165,166), which is very stressful when
seriously impeding respiration, has long been associated with
hypomagnesemia. Itevokes adrenergic and corticosteroid secretion,
which lower tissue Mg levels (supra vide) and it is characterized
by histamine release, which has been correlated with
hypomagnesemia (supra vide). It is noteworthy that the toxicity
of theophylline- a phosphodiesterase inhibitor that lowers
myocardial Mg levels, is intensified by beta-adrenergic agonists
and CS (167), and resembles the effects of experimental Mg
depletion: early tremor, later convulsions, and tachycardia,
arrhythmia, myocardial necrosis and death (168-170). The
favorable effect of Mg in counteracting toxic theophylline
reactions has been attributed to the Ca-blocking effect of Mg
(168,169), and to the membrane-stabilizing effect of Mg (169). In
addition to counteracting adverse effects of standard drug
therapy of asthma, Mg solutions (i.v. or aerosol) have been
reported effective, at intervals from 1934 to 1973 (165,171-173),
and more frequently in the past ten years (168,169,174-190).
Since the early anecdotal reports, there have been case reports
on the ameliorative and even life-saving effect of Mg in
intractable asthma (179,182-184,186-188) and favorable double
blind studies (174,180). The usefulness of Mg in bronchial
asthma, however, has been disputed (191-193). When effective for
bronchodilatation and to reduce bronchial reactivity to
administered histamine (justifying its adjunctive use with
standard drug therapy), doses of Mg sufficient to achieve
pharmacologic serum Mg levels were usually used
(176-183,185-188,194-196). The need for higher than physiologic
levels of Mg to counteract bronchial spasms induced in vitro had
been shown by Classen et al (197) for serotonin, acetyl choline
or histamine, and by Spivey et al (198) and Lindeman et al (199)
for bethanechol, electrical stimulation or histamine. Pertinent
to Mg and stress interrelationships in this disease, is the
increased stress hormone secretion effected by low Mg/Ca ratios
(supra vide). Mathew and Altura (200) have suggested that Mg
should have value as an adjunct in treating asthma because it
modulates smooth muscle contraction through its Ca-blockage or
competition. In their recent review, Landon and Young (201) point
out that the increased dietary Ca/Mg ratio of recent years, and
loss of Mg caused by diuretics and other drugs, may be a factor
in bronchial asthma, in view of Ca-binding to sites that increase
acetyl choline release which initiates smooth (bronchial) muscle
contraction.
ATHLETIC STRESS,
PERFORMANCE AND MAGNESIUM
Magnesium Deficiency and Supplementation in
Strenuously Exercised Animals
A series of studies by Keen and Lowney et al (202-206) with
rats, fed diets providing 50 and 100 ppm and half the adequate
intake of 400 ppm for 22 days, determined their endurance when
they were run on a treadmill to exhaustion. They showed that
decreased exercise capacity can be an early effect of Mg
deficiency. Analysis of plasma and muscle levels of Mg of rats
that had decreased endurance, sacrificed 2 days or immediately
after the stress test, showed slightly lower plasma Mg at both
times, and markedly lower muscle Mg immediately after exhaustion
(203). Mineral water containing 85 ppm Mg restored endurance
(202). Treadmill exercised, Mg deficient rats were shown by
Laires et al (207) to have reduced exercise capacity, increased
plasma lactate and FFA levels, and decreased plasma and rbc Mg
levels. In a test of endurance with the stress of fear (of
drowning), Hirneth and Classen (208) showed that Mg intake had to
be increased ten-fold (to 4000 ppm) in rats made to swim with a
weight attached to their tails, to significantly improve duration
of swimming before submersion.
Human Endurance Exercise, Magnesium, and
Metabolism
Refsum et al (210), in 1973, attributed transiently lowered
serum Mg and slightly increased rbc Mg, of cross-country skiing
racers, to shift of Mg from e.c. to i.c. fluid. Well trained
athletes were found by Casoni et al (210) to have significantly
increased mean rbc Mg and slightly decreased serum Mg after a
25-km marathon, compared to pre-race levels. Serum Mg decreased
and rbc Mg increased in 40 trained long-distance runners, 19 to
71 years of age, studied by Hoffmann and Boehmer (211) after each
of two 25 km marathon races. Lijnen et al (212) found lower rbc
and plasma Mg in teen-aged marathon runners just after the race
than before, that returned to pre-race values 12 hours later.
Urinary Mg decreased immediately after the race, and increased 12
hours later. Joborn et al found that long-term steady state
exercise had little effect on Mg levels, but there was a decrease
during the first hour of recovery (20).
High altitude intensive training resulted in negative Mg
balance, in Mader et al's study (213). Hypomagnesemia sufficient
to cause convulsions was seen in a man subjected to 4 hours
intense exertion under conditions of heat (Jooste et al [214]).
Non-supplemented marathoners lost muscle protein (Bertschat et al
[215]).
Important studies of long-term Mg loss by trained adults
undergoing sustained heavy exercise have been reported by
Stendig-Lindberg et al (216-219). Apparently healthy young
subjects who underwent a 7-month graded physical training program
before undertaking a 120-km march (of 22 hours duration) had Mg
intakes of 340 mg in food and 364 mg Mg in water (216). Despite
the higher than usual intake, the mean serum Mg pre-march (1.66
mEq/L) fell significantly 1 hour after the march ended to
(1.4mEq/L). After rising to almost pre-march levels at 24 hours,
it fell again, at 72 hours to 1.3 mEq/L; 89% had hypomagnesemia.
They exhibited elevated serum creatine kinase (CK) activity,
suggesting that the serum Mg rise at 24 hrs resulted from
exertional rhabdomoyolysis or loss of membrane integrity.
Significantly lowered serum Mg (1.51 mEq/L) persisted for 3
months (217). A study after a 70 km march extended the findings
to aspartate amino transferase (S-AST), alanine amino transferase
(S-ALT), creatine kinase activity (S-CK), and VO2 ml/min-1. kg-1
(VO2 max) (218). Maximal aerobic power, hemoglobin, hematocrit,
total protein and albumin were unchanged throughout. Immediately
after the march, serum Mg did not change, but S-AST, S-ALT, hours
after the march, serum Mg fell significantly; it remained low
after 18 days, with no intervening marches or dietary changes.
The means of S-ALT and S-CK rose & S-CK rose slightly. At
72significantly. For the first time there was a significant rise
of blood sugar, and of serum triglycerides, and a second rise of
total cholesterol. In a long-term follow-up of two additional
groups of young men subjected to the same training and 120 km
march as reported in 1985 (260), significant depression of serum
Mg persisted as long as 10-11 months after the march in the two
test groups; serum triglycerides showed a delayed rise (219).
Short-term Intensive Exercise and Magnesium
Plasma Mg was unchanged, but rbc Mg decreased significantly
during exercise on an ergometer in a study by Golf et al (10).
Boehmer (220), however found that an hour's strenuous exercise
sharply lowered serum Mg in a large group of well-trained
athletes, whose resting serum Mg values were normal. On the other
hand, increased plasma Mg, attributed to reduction of plasma
volume and influx of Mg to the vascular pool during short-term
intense exercise on a bicycle ergometer, was reported by Joborn
et al (19) and Ansquer (221), who commented that when there was a
fall in serum Mg, it was accompanied by increased rbc Mg, the
total blood Mg remaining constant, indicating an
intercompartmental shift. Treadmill running until exhaustion was
shown by Deuster et al (222) to transiently decrease plasma Mg
significantly, with over 85% of the loss caused by shift to rbc.
There was significantly increased urinary Mg on exercise, and of
post-exercise blood lactate and oxygen consumption during
recovery versus a control period. Exercise-induced
inter-compartmental Mg shifts in blood Mg returned to
pre-exercise values in 2 hours; urinary Mg loss on the exercise
day returned to baseline the next day. They suggested that the
exercise-induced loss of urinary Mg might depend on exercise
intensity and relative contribution of anaerobic metabolism to
total energy expended during exertion. Lukaski et al (223), who
treadmill-tested 44 healthy male university athletes and 20
untrained men, observed that athletes' average maximum oxygen
consumption was greater than in non-athletes, and was
significantly correlated with plasma Mg, suggesting that Mg may
enhance O2 delivery to working muscles in trained subjects. Conn
et al (224) found that VO2max was significantly higher in
pre-adolescent swimmers than in controls for both sexes, despite
comparable plasma, rbc and whole blood Mg. Laires et al (225)
assessed the effect of swimming for 30 minutes on plasma Mg and
lipids in 6 well-trained swimmers before, just after, 30 minutes
after, and 24 hours after exercise. Serum Mg decreased
significantly shortly after exercise, returning to base line the
next day; rbc Mg did not change. Plasma total cholesterol
decreased significantly 30 minutes after exercise, with a
significant positive correlation between plasma Mg and plasma
HDL-cholesterol that disappeared after exercise but reappeared 24
hours later.
Magnesium Supplementation and Dietary Magnesium Intakes
of Athletes
Mg Supplements and Athletic Performance. The effect
of K + Mg aspartate supplements on the capacity for intense
exercise (90 minutes) was reported in 1968 by Ahlborg et al (226)
in six young men, the day before, and daily on four days of
testing on a bicycle ergometer until complete exhaustion and/or
muscle pain required stopping. Those supplemented on day 3
exhibited 50% increased work capacity before muscle pain
developed, versus those given placebo. It was postulated that Mg
accelerated glycogen synthesis or spared glycogen in muscle,
thereby sparing energy. That premise was justified by the
demonstrated fall in muscle glycogen and increase in lactate
after 20 minutes of heavy exercise on a bicycle ergometer,
without supplementation, shown by Bergstrom et al in 1971
(227).
Endurance (power) athletes under constant strain, given Mg
supplements by Boehmer in 1979 (228) did not exhibit the
declining Mg levels seen in the power athletes not so
supplemented, and had better performance and endurance. In the
study of de Haan et al (229), K and Mg aspartate supplementation
did not improve muscle performance during short intensive
exercise, as Vieth et al (230) had shown it to do in endurance
athletes. In a double-blind study of marathon racers, whom
Terblanche et al (231) described as Mg-replete, 365 mg/d of Mg
had no effect on performance or recovery. In another double-blind
study, of Mg supplemented athletes, Wodick and Grunert-Fuchs
(232) used a running board and bicycle ergometry and found that
480 mg of Mg as the aspartate-HCl salt/day for 4 weeks
significantly improved physical capacity. Four weeks of
supplementation with Mg aspartate-HCl was found to increase rbc
Mg and decrease maximal ventilation by 11% as compared to a
pre-Mg test in 14 male rowers in maximal-tests on a rowing
ergometer (Golf et al [233]). Plasma and urinary lactate
increased from 1 mM/l to 15 mM/l, and blood oxygen content
decreased.
The effect on blood coagulation and fibrinolytic factors of
supplementing male swimmers for a month with 480 mg of Mg/day or
with a placebo before a 1500 meter race was studied by Pohlmann
et al (234). Those receiving Mg had both increased serum and rbc
Mg levels, and showed anti-coagulating effects.
Dietary Mg Intake of Athletes
Surveys of dietary intakes of athletes have shown that as many
as half consumed diets delivering less than the 1980 RDA (235)
estimated for sedentary adults, which was lowered in the current
edition(110). Those undergoing active anabolism and/or subjected
to stress have substantially higher Mg needs. Studies of diets of
athletes have shown that such needs are commonly not met by their
diets (222,236-242). Supplementation of athletes to keep their Mg
status optimal, to ensure their best performance without
interfering muscle cramps and premature fatigue, and to prevent
muscle damage, has been recommended or implied by those reporting
improvement in the Mg-treated group in placebo-controlled studies
(supra vide). Since athletes undergo severe physical stress, as
well as the psychological drive to win, and most ingest
sub-optimal amounts of Mg, they are vulnerable to Mg deficiency.
Diets very rich in Mg, or prophylactic use of Mg supplements
should be advised to be sure of Mg intake of not less than 6 to
10 mg/kg/day, and possibly more (97,221,236-238,243-246).
Sudden death of pigs and cattle being transported under the
stressful conditions of crowding has been reduced in incidence by
Mg supplementation with Mg aspartate HCl or MgCl2 in sufficient
dosage to raise serum Mg (247). Calmer behavior of Mg treated
animals was observed in 49.5% of groups during fattening and in
37.6% during transport and in the slaughterhouse. (248,249). Up
to half of losses of young chickens (broilers) have been
attributed to a sudden death syndrome, possibly from stress. A
study of 5180 broilers whose high protein and carbohydrate diets
were supplemented with Mg aspartate HCl, for 40 days during their
fattening period, disclosed significant reduction of losses from
sudden death (250).
Stress-intensification of Mg inadequacy may well be to blame
for the SCD associated with stress, even in young healthy
athletes. This report has evaluated data implicating high Ca/Mg
ratios in increased adverse responses to stress. Stress hormone
intensification of Mg loss, and stimulation of their secretion by
high Ca/Mg constitute a self-reinforcing loop. High Ca/Mg ratios
lead to increased blood coagulation and vasotonus, which are also
influenced by prostaglandins and fibronectin, that are affected
by low Mg levels. It is provocative that pharmacologic doses of
Mg, are therapeutic in conditions that are intensified by loss of
Mg, and in which Mg inadequacy might well be a contributory
factor. High serum Mg levels, such as are required to control
eclampsia, have been shown to be effective adjunctive therapy in
bronchial asthma unresponsive to standard therapy; prompt high
dosage i.v.Mg has prevented or reduced the incidence of post-AMI
complications and significantly improved survival in double-blind
trials (251-256).
Worth consideration and trial is the possibility that higher
than usual Mg intake - whether from a Mg-rich diet or from
supplementing the usual diet with Mg salts, possibly with
anti-oxidant nutrients, might be protective against damage caused
by the usual vicissitudes of life and unusual stresses. Might
higher Mg (and vitamin E) intakes decrease the risk of
arrhythmias and SCD in humans? Large intervention studies, like
those undertaken to elucidate the effects of lowering saturated
fat intake on cardiovascular disease and cancer, are needed to
determine the extent to which adding Mg (and anti-oxidant
nutrients) to already recommended dietary changes, might further
improve health and increase stress-tolerance. Such studies are
particularly urgent in view of the recent National Institute of
Health recommendation (257) that the optimum Ca intake be further
increased to 1500 mg/day (to prevent osteoporosis), which
disregards the low American Mg intake, less than 300 mg/day. This
would bring the Ca/Mg ratio to 5/1 - which is above the 4/1 ratio
of Finland, the land with the highest ischemic heart disease
death rate for young to middle-aged men (258,259).
1. Cannon WB: Bodily Changes in pain, hunger, fear, and rage.
Publ Appleton Century, NY, NY, 1920.
2. Raab W: Cardiotoxic effects of emotional, socioeconomic,
and environmental stresses. In RECENT ADVANCES IN STUDIES ON
CARDIAC STRUCTURE AND METABOLISM I, Myocardiology, eds E. Bajusz,
& G. Rona, University Park Press, Baltimore, MD, pp. 707-713,
1972.
3. Raab W: Sudden death of a young athlete with an excessive
concentration of epinephrine-like substances in the heart muscle.
Arch Path 36:488-492, 1943.
4. Raab W: Myocardial electrolyte derangement: crucial feature
of pluricausal so-called coronary heart disease. Ann NY Acad Sci
147:629-686, 1969.
5. Braunwald E, Harrison DC, Chidsey CA (Editorial): The heart
as an endocrine organ. Am J Med 36:1-4, 1964.
6. Shahab L, Wollenberger A, Krause EG, Genz S: Effect of
acute ischaemia on catecholamines and cyclic AMP levels in normal
and hypertrophied myocardium. in EFFECT OF ACUTE ISCHEMIA ON
MYOCARDIAL FUNCTION, eds MF Oliver, DG Julian, & KW Donald,
Churchill Livingstone Press, London, UK, pp 97-107, 1972.
7. Mason JW, Mangan G, Brady JV, Conrad D, Rioch D McK:
Concurrent plasma epinephrine, norepinephrine, and
17-hydroxycorticosteroid levels during conditioned emotional
disturbances in monkeys. Psychosom Med 23:344-000, 1961.
8. Basowitz H, Persky H, Korchin SJ, Grinker RR: Anxiety and
stress. Blakiston Div, McGraw-Hill Book Co, NY, NY, 1955.
9. Persky H: Adrenocortical function during anxiety. in
PHYSIOLOGIC CORRELATES OF PSYCHOLOGICAL DISORDERS, eds R Roessler
& NS Greenfield, Univ of Wisconsin Press, Madison
WI,1962.
10. Golf SW, Happel O, Graef V, Seim KE: Plasma aldosterone,
cortisol and electrolyte concentrations in physical exercise
after magnesium supplementation. J Clin Chem Clin Biochem
22:717-721, 1984.
11. Douglas WW, Rubin RP: The effects of alkaline earths and
other divalent cations on adrenal medullary secretion. J Physiol
175:231-241, 1964.
12. Boullin DJ: The action of extracellular cations on the
release of the sympathetic transmitter from peripheral nerves. J
Physiol 189:85-99, 1967.
13. Kirpekar SM, Misu Y: Release of noradrenalin by splenic
nerve stimulation and its dependence on calcium. J Physiol
188:219-234, 1967.
14. Euler US v, Lishajko F: Effects of Mg2+ and Ca2+ on
noradrenaline release and uptake in adrenergic nerve granules in
different media. Acta Physiol Scand 89:415-422, 1973.
15. Baker PF, Rink TJ: Catecholamine release from bovine
adrenal medulla in response to maintained depolarization. J
Physiol 253:593-620, 1975.
16. Iseri LT, French JH: Magnesium: nature's physiologic
calcium blocker. Am Heart J 108:188-193, 1984.
17. Whyte KF, Addis GJ, Whitesmith R, Reid JL: Adrenergic
control of plasma magnesium in man. Clin Sci 72:135-138, 1987
18. Johansson BW, Juul-Moller S, Ruter G, Soes-Pedersen U:
Effect of i.v. adrenaline infusion on serum electrolytes,
including S-Mg. Magnesium Bull 8:259-260, 1986.
19. Joborn H, Akerstrom G, Ljunghall S: Effects of exogenous
catecholamines and exercise on plasma magnesium concentrations.
Clin Endocrinol Oxford 23:219-226, 1985.
20. Fletcher GF, Sweeney ME, Fletcher BJ: Blood magnesium and
potassium alterations with maximal treadmill exercise testing:
effects of beta-adrenergic blockade. Am Heart J 121 (Pt
1)):105-110, 1991.
21. James MF: Use of magnesium sulphate in the anaesthetic
management of phaeochromocytoma: a review of 17 anaesthetics. Br
J Anaesth 62:616-623, 1989.
22. James MF, Beer RE, Esser JD: Intravenous magnesium sulfate
inhibits catecholamine release associated with tracheal
intubation. Anesth Analg 68:772-776, 1989.
23. Milewicz A: An attempt at evaluation magnesium metabolism
in experimental hyperadrenalaemia in rabbits and in cases of
phaeochromocytoma treated surgically. Materia Med Pol 4:267-271,
1978.
24. Cohen L, Kitzes R: Pheochromocytomaa rare cause of
hypermagnesemia. Magnesium 4:165-167, 1985.
25. Balazs T: Cardiotoxicity of isoproterenol inexperimental
animals. Influence of stress, obesity and repeated dosing. in
RECENT ADVANCES IN STUDIES ON CARDIAC STRUCTURE AND METABOLISM.
I. MYOCARDIOLOGY, eds E Bajusz & G Rona, University Park
Press, Baltimore MD, pp 770-778, 1972.
26. Franz KB, Rueddel H, Todd GL, Dorheim TA, Buell JC, Eliot
RS: Physiologic changes during a marathon, with special reference
to magnesium. J Am Coll Nutr 4:187-194, 1985.
27. Lutwak L, Hurt C, Reid JM: Effect of corticoids on
magnesium metabolism in man. Clin Res 9:184, 1961.
28. Hanna S, MacIntyre: The influence of aldosterone on
magnesium metabolism. Lancet 2:348-350, 1960.
29. Care AD, Ross DB: The role of the adrenal cortex in
magnesium homeostasis in the aetiology of hypomagnesaemia. Res
Vet Sci 4:24-63, 1963.
30. Mader IJ, Iseri LT: Spontaneous hypopotassemia,
hypomagnesemia, alkalosis and tetany due to hypersecretion of
corticosterone-like mineralocorticoid. Am J Med 19:976-988,
1955.
31. Horton R, Biglieri EG: Effect of aldosterone on the
metabolism of magnesium. J Clin Endocrin Metab 22:1187-1192,
1962.
32. Cantin M: Relationship of juxtaglomerular apparatus and
adrenal cortex to biochemical and extracellular fluid volume
changes in magnesium deficiency. Lab Invest 22:558-568, 1970.
33. Care AD, McDonald IR: Plasma magnesium concentration as a
possible factor in the control of aldosterone secretion. Biochem
J 87:2P-3P,1963.
34. Ryan MP, Ryan MF, Counihan TB: The effect of diuretics on
lymphocyte magnesium and potassium. Acta med Scandinav Suppl
647:153-161, 1981.
35. Widman L, Dyckner T, Wester PO: Effect of moduretic and
aldactone on electrolytes in skeletal muscle in patients on
long-term diuretic therapy. Acta med Scandinav Suppl 661:33-35,
1982.
36. Bajusz E, Selye H: Conditioning factors for cardiac
necrosis. Trans N Y Acad Sci Ser II 1:659-667, 1959.
37. Chappel CI, Rona G, Gaudry R: The influence of adrenal
cortical steroids on cardiac necrosis produced by isoproterenol.
Acta Endocr 32: 419-424, 1959.
38. Bajusz E: The terminal electrolyte-shift mechanism in
heart muscle; its significance in the pathogenesis and prevention
of necrotizing cardiomyopathies. in ELECTROLYTES AND
CARDIOVASCULAR DISEASE. I. FUNDAMENTAL ASPECTS. ed E Bajusz,
Williams & Wilkins Press, Baltimore MD, pp 274-322, 1965.
39. Rona G, Kahn S, Chappel CI: The effect of electrolytes on
experimental infarct-like myocardial necrosis. IBID, pp
181-191.
40. Lehr D: The role of certain electrolytes and hormones in
disseminated myocardial necrosis. IBID, pp 248-273.
41. Selye H: The evolution of the stress concept. Am J Cardiol
26:289-299, 1970.
42. Seelig MS, Heggtveit HA: Magnesium interrelationships in
ischemic heart disease. A review. Am J Clin Nutr 27:59-79,
1974.
43. Vormann J, Guenther T, Leder O: Effect of isoproterenol
and phenylephrine on the heart of normal and Mg-deficient rats.
Magnesium Bull 3:130-134, 1981.
44. Guenther T: Cardiovascular biochemistry. Magnesium Bull
8:136-144, 1986.
45. Vormann J, Fischer G, Classen HG, Thoni H: Influence of
decreased and increased magnesium supply on the cardiotoxic
effects of epinephrine in rats. Arzneimittelforschung 33:205-210,
1983.
46. Ebel H, Guenther T: Role of magnesium in cardiac disease.
J clin Chem clin Biochem 21:249-265, 1983.
47. Classen HG, Fischer G, Jacob R, Marx J, Schimatschek H,
Stein C: Prenecrotic electrolyte alterations of the adrenergic
cardiopathy: Potentiation by magnesium depletion and prevention
by high dietary magnesium levels and verapamil. Magnesium Bull
8:82-92, 1986.
48. Bloom S: Effects of magnesium deficiency on the
pathogenesis of myocardial infarction. Magnesium 5:154-164,
1986.
49. Bloom S: Magnesium deficiency cardiomyopathy. Am J
Cardiovasc Pathol 2:7-17, 1988.
50. Vormann J, Forster R, Guenther T, Ebel H:
Lipolysis-induced magnesium uptake into fat cells. Magnesium Bull
5:39-41, 1983.
51. Rayssiguier Y: Hypomagnesemia resulting from adrenaline
infusion in ewes: its relation to lipolysis. Horm Metab Res
9:309-314, 1977.
52. Flink EB, Shane SR, Scobbo RR, Blehschmidt NG, McDowell P:
Relationship of free fatty acids and magnesium in ethanol
withdrawal in dogs. Metabolism 28:858-865, 1979.
53. Oliver MF, Kurien VA, Greenwood TW: Relation between serum
free fatty acids and arrhythmias and death after acute myocardial
infarction. Lancet 1:710-715, 1962.
54. Opie LH: Metabolism of free fatty acids, glucose and
catecholamines in acute myocardial infarction. Relation to
myocardial ischemia and infarct size. Am J Cardiol 36:938-953,
1975.
55. Flink EB, Brick JE, Shane SR: Alterations of long-chain
free fatty acid and magnesium concentrations in acute myocardial
infarction. Arch Intern Med 141:441-443, 1981.
56. Henrotte JG, Plouin PF, Levy-Leboyer C, Moser G,
Sidoroff-Girault N, Franck G, Santarromana M, Pineau M: Blood and
urinary magnesium, zinc, calcium, free fatty acids, and
catecholamines in type A and type B subjects. J Am Coll Nutr
4:165-172, 1985.
57. Pratt K, Moody ML, Cinlee RK, Rueddel H, Franz KB: Changes
in serum free fatty acids and magnesium during a marathon.
Magnesium 4:207-208, 1985.
58. Durlach J: [Physiologic antithrombotic role of magnesium.
Concerning magnesium deficient phlebothrombotic disease.] Coeur
Med Interne 6: 213-232, 1967. (in French)
59. Seelig MS: MAGNESIUM DEFICIENCY IN THE PATHOGENESIS OF
DISEASE. EARLY ROOTS OF CARDIOVASCULAR, SKELETAL, AND RENAL
ABNORMALITIES. Plenum Medical Book Co, NY, NY 1980.
60. Kurgan A, Gertz D, Wajnberg RS, Eldor A, Jersky J, Nelson
E: Effect of magnesium sulfate on platelet deposition in rabbits
following temporary arterial occlusion with surgical clips.
Surgery 87:390-396, 1980.
61. Gertz SD, Wajnberg RS, Kurgan A, Uretzky G: Effect of
magnesium sulfate on thrombus formation following partial
arterial constriction: implications for coronary vasospasm.
Magnesium 6:225-235, 1987.
62. Sos J: An investigation into the nutritional factors of
experimental cardiopathy. in ELECTROLYTES AND CARDIOVASCULAR
DISEASE I. FUNDAMENTAL ASPECTS. ed E Bajusz, Williams &
Wilkins Press, Baltimore MD, pp 161-180, 1965.
63. Rigo J: The relationship between magnesium and the
vascular system. in Proc 1st Intl Mg Sympos, Vittel, France I. ed
J Durlach, pp 215-228, 1971. 64. Rishi M, Ahmad A, Makheja A,
Karcher D, Bloom S: Effects of reduced dietary magnesium on
platelet production and function in hamsters. Lab Invest
63:717-721, 1990.
65. Seelig MS: Increased need for magnesium with the use of
combined oestrogen and calcium for osteoporosis treatment.
Magnesium Res:3:197-215, 1990.
66. Seelig MS: Interrelationship of magnesium and estrogen in
cardiovascular and bone disorders, eclampsia, migraine and
premenstrual syndrome. J Am Coll Nutr 12:442-458, 1993.
67. Nigam S, Averdunk R, Guenther T: Alteration of prostanoid
metabolism in rats with magnesium deficiency. Prostaglandins
Leukotrienes Med 23:1-10, 1986.
68. Nadler JL, Goodson S, Rude RK: Evidence that prostacyclin
mediates the vascular action of magnesium in humans. Hypertension
9:379-383, 1987.
69. Nadler J, Kuong H, Ehrlich L, Ryzen E, Frances R, Rude R:
Magnesium plays an important role in the regulation of
thromboxane release and platelet aggregation. 1989 Natl Mtg of Am
Fed Clin Res, Washington DC, 1989.
70. Nadler J, Ilwang D, Yen C, Rude R: Magnesium plays a key
role in inhibiting platelet aggregation and release reaction. Am
Heart Assoc Sci Session, Anaheim CA, 1991.
71. Woods KL: Possible pharmacological actions of magnesium in
acute myocardial infarction. Brit Clin Pharmacol 32:3-10,
1991.
72. Altura BM, Altura BT, Waldemar Y: Effects of magnesium on
prostaglandin responses in arterial and venous smooth muscle. in
MAGNESIUM IN HEALTH AND DISEASE. eds M Cantin, MS Seelig, (Proc
of 2nd Intl Mg Sympos, Quebec, 1976), Spectrum Press, NY, pp
687-694, 1980.
73. Bergman G, Daly K, Atkinson L, Rothman M, Richardson PJ,
Jackson G, Jewett DE: Prostacyclin: haemodynamic and metabolic
effects in patients with coronary artery disease. Lancet
1:569-572, 1981.
74. Briel RC, Lippert TH, Zahradnik HP: [Changes in blood
coagulation, thrombocyte function and vascular prostacyclin
synthesis caused by magnesium sulfate]. Geburtsh Frauenheilkd
47:332-336, 1987. (in German)
75. Watson KV, Moldow CF, Ogburn PL, Jacob HS: Magnesium
sulfate: rationale for its use in preeclampsia. Proc Natl Acad
Sci USA 83:1075-1078, 1986.
76. Mak IT, Weglicki WB: Comparative antioxidant activities of
propranolol, nifedipine, verapamil, and diltiazem against
sarcolemmal membrane lipid peroxidation. Circulation Res
66:1449-1452, 1990.
77. Weglicki WB, Mak IT, Simic MG: Mechanisms of
cardiovascular drugs as antioxidants. J Mol Cell Cardiol
22:1199-1208, 1990.
78. Freedman AM, Atrakchi AH, Cassidy MM, Weglicki WB:
Magnesium deficiency-induced cardiomyopathy: protection by
vitamin E. Biochem Biophys Res Commun 170:1102-1106, 1990.
79. Freedman AM, Cassidy MM, Weglicki WB: Magnesium-deficient
myocardium demonstrates an increased susceptibility to an in vivo
oxidative stress. Magnes Res 4:185-189, 1991.
80. Weglicki WB, Freedman AM, Bloom S, Atrakchi AH, TM,
Cassidy MM, Dickens BJ: Antioxidants and the cardiomyopathy of Mg
Deficiency. Am J Cardiovasc Path 4:210-215, 1992.
81. Mak IT, Boehme P, Weglicki WB: Antioxidant effects of
calcium channel blockers against free radical injury in
endothelial cells. Circulation Res 70:1099-1103, 1992.
82. Atrakchi A, Weglicki W B, Bloom S: Beta blockers protects
against magnesium-deficiency-induced cardiac injury. FASEB J
3:A921, 1989.
83. Atrakchi AH, Bloom S, Dickens BF, Mak IT, Weglicki WB:
Hypomagnesemia and isoproterenol cardiomyopathies: protection by
probucol. Cardiovasc Pathol 1:155-160, 1992.
84. Singal PK, Kapur N, Dillon KS, Beamish RE, Dhalla NS: Role
of free radicals in catecholamine-induced cardiomyopathy. Can J
Physiol Pharmacol 60:1390-1397, 1982.
85. Singh RB, Rastogi SS, Ghosh S, Niaz MA: Dietary and serum
magnesium levels in patients with acute myocardial infarction,
coronary artery disease and noncardiac diagnoses. J Am Coll Nutr
13:139-143, 1994.
86. Singh RB, Sircar AR, Rastogi SS, Garg V: An Indian
experiment with nutritional modulation in acute myocardial
infarction. Am J Cardiol 69:879-885, 1992.
87. Whang R, Wagner R: The influence of venous occlusion and
exercise on serum magnesium concentration. Metabolism 15:608-612,
1966.
88. Engel RR, Elin RJ: Hypermagnesemia from birth asphyxia, J
Pediatrics 77:631-637, 1970.
89. Nayler WG: The heart cell: some metabolic aspects of
cardiac arrhythmias. Acta med Scand Suppl 647:17-31, 1981.
90. Van Vliet PD, Burchell HB, Titus JL: Focal myocarditis
associated with pheochromocytoma. New Engl J Med 27:1102-1108,
1966.
91. Ferrans VJ, Hibbs RG, Cipriano PR, Buja LM: Histochemical
and electron microscopic studies of norepinephrine-induced
myocardial necrosis in rats. In RECENT ADVANCES IN STUDIES ON
CARDIAC STRUCTURE AND METABOLISM. I, Myocardiology eds E Bajusz,
& G Rona, University Park Press, Baltimore, MD, pp 495-525,
1972.
92. Lehr D, Blaiklock R, Brown A, Dapson S: Magnesium loss as
a reliable indicator of acute myocardial injury. in MAGNESIUM IN
HEALTH AND DISEASE, eds M Cantin, MS Seelig, (Proc of 2nd Intl Mg
Sympos, Quebec, 1976), Spectrum Press, NY. pp 499-506, 1980.
93. Smetana R, Brichta A, Glogar D, Meisinger V,
Gotsauner-Wolf M, Spona J: Magnesium profile under stress
conditions in coronary artery disease. Magnesium Res 2:41-42.
1989
94. Jenkins CD: The coronary prone personality. in
PSYCHOLOGICAL ASPECTS OF ASPECTS OF MYOCARDIAL INFARCTION AND
CORONARY CARE, ed W Gentry, CV Mosby Press, St. Louis MO, pp
5-21, 1975.
95. Henrotte JG: Type A behavior and magnesium metabolism.
Magnesium 5:201-210, 1986.
96. Henrotte JG: Recent advances on genetic factors regulating
blood and tissue magnesium concentrations; relationship with
stress and immunity. In Itokawa Y, Durlach J (eds): MAGNESIUM IN
HEALTH AND DISEASE, eds Y. London: John Libbey Press, pp.
285-289, 1989.
97. Durlach J: MAGNESIUM IN CLINICAL PRACTICE. (transl by D
Wilson), John Libbey & Co, London, UK, 1988.
98. Fehlinger R: Therapy with magnesium salts in logical
diseases. A critical appraisal. Magnesium Bull 12:35-42,
1990.
99. Durlach J, Durlach V: Idiopathic mitral valve prolapse and
magnesium. State of the art. Magnesium Bull 8:156-169, 1986.
100. Galland LD, Baker SM, McLellan RK: Magnesium deficiency
in the pathogenesis of mitral valve prolapse. 5:165-174,
1986.
101. Schimatschek HF, Hickl R, Classen HG: [Magnesium,
calcium, and phosphorus levels in the serum of children with
functional disorders. Preliminary data of a multicenter
epidemiologic study with special emphasis on magnesium.]
Magnesium Bull 10:105-113, 1988. (in German)
102. Schroeder HA: HYPERTENSIVE DISEASES. CAUSES AND CONTROL.
Lea & Febiger Press, Philadelphia, PA, 1953.
103. Resnick LM, Laragh JH, Sealey JE, Alderman MH: Divalent
cations in essential hypertension. Relations between serum
ionized calcium, magnesium and plasma renin activity. N Engl J
Med 309:888-891, 1983.
104. Resnick LM, Gupta RK, Laragh JH: Intracellular free
magnesium in erythrocytes of essential hypertension: relation to
blood pressure and serum divalent cations. Proc Natl Acad Sci USA
81:6511-6515, 1984.
105. Resnick LM, Laragh JH: Renin, calcium metabolism and the
pathophysiologic basis of antihypertensive therapy. Am J Cardiol
56:68H-74H, 1985.
106. Resnick L, Gupta R, Gruenspan H, Laragh J: Does decreased
intracellular free magnesium mediate the peripheral insulin
resistance of hypertension and obesity? Endocr Soc Sympos,
1988.
107. Resnick LM, Gupta RK, Sosa RE, Corbett ML, Sealey JE,
Laragh JH: Effects of altered dietary calcium intake in
experimental hypertension - Role of intracellular free magnesium.
J Hypertension 4 (Suppl 5):S182-S185, 1986.
108. Rueddel H, Baehr M, Schaechinger H, Schmieder R, Ising G:
Positive effects of magnesium supplementation in patients with
labile hypertension and low magnesium concentration. Magnesium
Bull 11:93-98, 1989.
109. Kuti V: A study of some clinical effects of chronic Mg
supplementation in humans. Magnesium Res 2:229, 1989.
110. Food and Nutrition Board, Natl Res Council: RECOMMENDED
DIETARY ALLOWANCES. 10th Ed, Washington, DC, Natl Acad Press. pp
187-194, 1989.
111. Raab W, Schroeder G, Wagner R, Gigee W, Adams MC, Diorio
NG, Feely DA, Starczewski YK, White P: Vascular reactivity and
electrolytes in normal and toxemic pregnancy. J Clin Endocrin
Metab 16: 1196-1216, 1956.
112. Zuspan FP: Adrenal gland and sympathetic nervous system
response in eclampsia. Am J Obstet Gynecol 114:304-313, 1972.
113. Seelig MS, Altura BT, Resnick LM, Handwerker SM, Altura
BM: Low magnesium, a common denominator in pathologic processes
in diabetes mellitus, cardiovascular disease, and eclampsia.
abstr: Plenary Session presentation, 33rd Ann Sympos of ACN, J Am
Coll Nutr 11:608, 1992.
114. Weaver K: A possible anticoagulant effect of magnesium in
pre-eclampsia. in MAGNESIUM IN HEALTH AND DISEASE. eds M Cantin,
MS Seelig, (Proc of 2nd Intl Mg Sympos, 1976), NY, Spectrum
Press. pp 833-838, 1980.
115. Weaver K: Pregnancy-induced hypertension and low birth
weight in magnesium-deficient ewes. Magnesium 5:191-200,
1986.
116. Weaver K: Magnesium and fetal growth. Tr Subst in
Environm Health 22:136-142, 1988.
117. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA,
McLaughlin MK: Preeclampsia: an endothelial cell disorder. Am J
Obstet Gynecol 161:1200-1204, 1989.
118. Calvin S, Weinstein L, Witte MH, Finley PR: Plasma levels
of fibronectin and prostacyclin metabolite in peripartum
preeclamptic women. Am J Perinatol 7:125-129, 1990.
119. Massow S, Fehlinger R, Seidel K, Hecht K, Glatzel E:
[Influence of sub-optimal Mg of rats during gestation and
lactation on stress sensitivity of their adult offspring.]
Magnesium Bull 4:182-188, 1982. (in German)
120. Miller JK, Schneider MD, Ramsey N, White PK, Bell MC:
Effects of hypomagnesemia on reactivity of bovine and ovine
platelets: possible relevance to infantile apnea and sudden
infant death syndrome. J Am Coll Nutr 9:58-64, 1990.
121. Caddell JL: Magnesium therapy in premature neonates with
apnea neonatorum. J Am Coll Nutr 7:5-16, 1988. [erratum in J Am
Coll Nutr 7:189,1988].
122. Caddell JL: Reply to an editorial concerning magnesium
therapy in premature infants with apnea neonatorum. J Am Coll
Nutr 7:185-191, 1988.
123. Caddell JL: Magnesium therapy in infants with
postneonatal apnea. Magnesium 7:103-116, 1988.
124. Caddell JL: Hypothesis: the role of magnesium deficiency
in the idiopathic respiratory distress syndrome of prematurity;
links between RDS and sudden infant death syndrome. abst.,
presentation at 33rd Annual ACN symposium, J Am Coll Nutr 11:627,
1992.
125. Altura BT, Altura BT, Carelle A, Prasad Turlapaty DMV:
Hypomagnesemia and vasoconstriction: possible relationship to
etiology of sudden death ischemic disease and hypertensive
vascular disease. Artery 9:212-213, 1981.
126. Altura BM, Altura BT: New perspectives on the role of
magnesium on the pathophysiology of the cardiovascular system. I.
Clinical aspects. Magnesium 4:226-244, 1985.
127. Seelig MS: Cardiovascular consequences of magnesium
deficiency and loss; pathogenesis, prevalence and manifestations
- magnesium and chloride loss in refractory potassium repletion.
Am J Cardiol 63:4G-21G, 1989.
128. Altura BM: Calcium antagonist properties of magnesium:
implication for antimigraine actions. (Editorial) Magnesium
4:169-175, 1985.
129. Swanson DR: Migraine and magnesium: eleven neglected
connections. Perspectives in Biol & Med 31:526-557, 1988.
130. Weaver K: Magnesium and migraine [letter]. Headache
30:168, 1990.
131. Rotton WN, Sachtleben MR, Friedman EA: Migraine and
eclampsia. Obstet Gynecol 14:322-330, 1959.
132. Hannington E: Migraine, a blood disorder. Lancet
2:501-503, 1978.
133. Ising H, Nawroth H, Guenther T: Accelerated aging of rats
by Mg deficiency and noise-stress. Magnesium Bull 3:142-145,
1981.
134. Franz K: Hearing thresholds of rats fed different levels
of magnesium for two weeks. Abstr, presentation at 33rd Annual
Sympos of ACN, J am Coll Nutr 11:612, 1992.
135. Ising H: Interaction of Noise-Induced Stress and
Magnesium. Artery 9:205-211, 1981.
136. Ising H, Handrock M, Guenther T, Fischer R, Combrowski:
Increased noise trauma in guinea pigs through magnesium
deficiency. Arch Otorhinolaryngol 236:139-146, 1982.
137. Guenther T, Ising H, Joachim Z: Biochemical mechanisms
affecting susceptibility to noise-induced hearing loss. Am J Otol
10:36-41, 1989.
138. Joachim Z, Babisch W, Ising H, Guenther T, Handrock M:
Dependence of noise-induced hearing loss upon perilymph magnesium
concentration. J Acoust Soc Am 7:104-108, 1983.
139. Heroux O, Peter D, Heggtveit A: Long-term effect of
suboptimal dietary magnesium on magnesium and calcium contents of
organs, on cold tolerance and on lifespan, and its pathological
consequences in rats. J Nutr 107:1640-1652, 1977.
140. Keys A, Brozek J, Henschel A et al: The BIOLOGY OF HUMAN
STARVATION. Univ Minnesota Press, Minneapolis, MN, pp 185-186,
200-202, 1950.
141. Zimmer R, Weill J, Dubois M: The nutritional situation in
the camps of the unoccupied zone of France in 1941 and 1942 and
its consequences. New Engl J Med 230:303-314, 1944.
142. Mellinghoff K, Van Lessen W: [Magnesium-calcium balance
in starvation.] Dtsche Arch klin Med 194:285-293, 1949. (in
German)
143. DeLeeuw IH, vanGaal L, Vanroelen W: Magnesium and
obesity: Magnesium and obesity; effects of treatment on magnesium
and other parameters. Magnesium 6:40-47, 1987.
144. Sunderman FW: Studies in serum electrolytes. XIV. Changes
in blood and body fluids in prolonged fasting. Am J Clin Path
17:169-180, 1947.
145. Consolazio CF, Matoush LO, Johnson HL, Nelson RA,
Krzywick HJ: Metabolic aspects of acute starvation in normal
humans (10 days). Am J Clin Nutr, 20:672-683, 1967.
146. Drenick EJ, Hunt IF, Swendseid ME(): Magnesium depletion
during prolonged fasting of obese males. J Clin Endocrinol
29:1341-1348, 1969.
147. Cubberley PT, Polster SA, Schulman CI: Lactic acidosis
and death after the treatment of obesity by fasting. New Engl J
Med 272:628-630, 1965.
148. Lindeman RD: Nutritional influences on magnesium
homeostasis with emphasis on renal factors. in MAGNESIUM IN
HEALTH AND DISEASE, eds M Cantin & MS Seelig, Spectrum Press,
NY (Proc of 2nd Intl Sympos on Magnesium, Quebec, 1976), pp
381-399, 1980.
149. Garnett ES, Barnard DL, Ford J, Goodbody RA, Woodehouse
MA: Gross fragmentation of cardiac myofibrils after therapeutic
starvation for obesity. Lancet 1:914-916, 1969.
150. Heggtveit HA, Herman L, Mishra RK: Cardiac necrosis and
calcification in experimental magnesium deficiency. Am J Path
45:757-782, 1964.
151. Michiel RR, Sneider JS, Dickstein RA, Hayman H, Eich RH:
Sudden death in a patient on a liquid protein diet. New Engl J
Med 198:1005-1007, 1978.
152. Frattali VP: Deaths associated with the liquid protein
diet. Natl Clearing House for Poison Control Cente rs Bull
23:4-11, 1979.
153. Licata AA, Lantigua R, Amatruda J, Lockwood D: Adverse
effects of liquid protein fast on the handling of magnesium,
calcium and phosphorus. Am J Med 71:767-772, 1981.
154. Licata AA: Magnesium handling during a liquid protein
fast. J Am Coll Nutr 2:315, 1983.
155. Classen HG: Stress and Magnesium. Artery 9:182-189,
1981.
156. Mangler B, Fischer G, Classen HG: The influence of
magnesium deficiency on the development of gastric stress ulcers
in rats. Magnesium Bull 4:9-12, 1982.
157. Classen HG: Stress and magnesium with special regard to
the gastrointestinal tract. in MAGNESIUM IN HEALTH AND DISEASE.
eds Y Itokawa, J Durlach, John Libbey Press, London, UK, pp
271-278, 1989.
158. Nowitzki S, Lehner M, Schimatschek HG, Classen HG:
[Marginal magnesium deficiency in children with acute abdominal
pain.] Magnesium Bull 10:114-118, 1988. (in German)
159. Mozsik G, Garamszegi M, Javor T, Nagy L, Patty I, Suto G,
Vincze A: A biochemical and pharmacological approach to the
genesis of ulcer disease II. A model study of stress-induced
injury to gastric mucosa in rats. Ann NY Acad Sci 597:264-281,
1990.
160. Wacker WEC: MAGNESIUM AND MAN. Harvard Univ Press,
Cambridge MA, 1980.
161. Guenther T: Biochemistry and pathobiochemistry of
magnesium. Artery 9:167-181, 1981.
162. Belanger LF, Van Erkel GA, Jakerow A: Behavior of the
dermal mast cells in magnesium deficient rats. Science 126:29-30,
1957.
163. Bois P, Gascon A, Beaulnes A: Histamine-liberating effect
of magnesium deficiency in the rat. Nature 197:501-502, 1963.
164. Hungerford GF: Role of histamine in producing the
eosinophilia of magnesium deficiency. Proc Soc Exp Biol Med
115:182-185, 1964.
165. Haury VG: Blood serum magnesium in bronchial asthma and
its treatment by the administration of magnesium sulfate. J Lab
Clin Med 26:340-344, 1940.
166. Chyrek-Borowska S, Obrzut D, Hofman J: The relation
between magnesium, blood histamine level and eosinophilia in the
acute stage of the allergic reactions in humans. Arch Immunol
Ther Exp Warsz 26:709-712, 1978.
167. Lehr D: Studies on the Cardiotoxicity of alpha- and
beta-adrenergic amines. in CARDIAC TOXICOLOGY, Ed T Balazs, Publ
CRC Press Inc, Boca Raton, FL. pp 75-112, 1981.
168. Chevalier B, le-Heuzey JY, Colombel B, Lavergne T, Guize
L, Rozensztajn L, Valty J: [Ventricular tachycardia during
theophylline overdose. Apropos of a case of reduction by
magnesium chloride.] Arch Mal Coeur 83:569-573, 1990. (in
French)
169. Golf SW, Enzinger D, Temme H, Graef V, Katz N, Roka L,
Friemann E, Lasch H-G, Friemann S, Tross H, Morr H: Effects of
magnesium in theophylline treatment of chronic obstructive lung
disease. in MAGNESIUM - A RELEVANT ION, eds B Lasserre & J
Durlach, Publ J Libbey, pp 443-451, 1991.
170. Shannon M: Predictors of major toxicity after
theophylline overdose. Ann Intern Med 119:1162-1167, 1993.
171. Rosello JC, Pla JC: [Magnesium sulfate in asthma crisis.]
Prensa Med Argent 23:1677-1680, 1936. (in Spanish)
172. Haury VG: The bronchodilator action of magnesium and its
antagonistic action (dilator action) against pilocarpine,
histamine, and barium chloride. J Pharmacol 64:58-64, 1938.
173. Vidal-Freyre A: [Magnesium and allergy.] In Proc 1st Intl
Mg Sympos, Vittel, France, 1971, Ed J Durlach, pp 415-416, 1973
(In French)
174. Brunner EH, Delabroise AM, Haddad ZH: Effect of
parenteral magnesium on pulmonary function, plasma cAMP, and
histamine in bronchial asthma. J Asthma 22:3-11, 1985
175. Rolla G, Bucca C, Bugiani M, Arossa W, Spinaci S:
Reduction of histamine-induced bronchoconstriction by magnesium
in asthmatic subjects. Allergy 42:186-188, 1987.
176. Rolla G, Bucca C, Caria E, Arossa W, Bugiani M, Cesano L,
Caropreso A: Acute effect of intravenous magnesium sulfate on
airway obstruction of asthmatic patients. Ann Allergy 61:388-391,
1988.
177. Okayama H, Aikawa T, Okayama M, Sasaki H, Mue S,
Takishima T: Bronchodilating effect of magnesium sulfate in
bronchial asthma. JAMA 257:1076-1078, 1988.
178. Rolla G, Bucca C, Caria E: Dose-related effect of inhaled
magnesium sulfate on histamine bronchial challenge in asthmatics.
Drugs Exp Clin Res 14:609-612, 1988.
179. McNamara RM, Spivey WH, Skobeloff E, Jacubowitz S:
Intravenous magnesium sulfate in the management of acute
respiratory failure complicating asthma. Ann Emerg Med
18:197-199, 1989.
180. Skobeloff EM, Spivey WH, McNamara RM, Greenspan L:
Intravenous magnesium sulfate for the treatment of acute asthma
in the emergency department. JAMA 262:1210-1213, 1989.
181. Rolla G, Bucca C, Caria E: The effect of magnesium on
bronchial hyperreactivity of asthmatics. Magnesium Res. 2:82,
1989. Abstr (5th Intl Mg Sympos, Kyoto, Japan, 8/8-12/88)
182. Rolla G, Bucca C: Hypomagnesemia and bronchial
hyperreactivity. A case report. Allergy 44:519-521, 1989.
183. Noppen M, Vanmaele L, Impens N, Schandevyl W:
Bronchodilating effect of intravenous magnesium sulfate in acute
severe bronchial asthma. Chest 97:373-376, 1990.
184. Rolla G, Bucca C, Bugiani M, Oliva A, Branciforte L:
Hypomagnesemia in chronic obstructive disease; effect of therapy.
Magnes Trace Elem 9:132-136, 1990.
185. Spivey WH, Greenspon L, McNamara R, Skobeloff EJ: JAMA
263:517, 1990. 186. Kuitert LM, Kletchko SL: Intravenous
magnesium sulfate in acute, life-threatening asthma. Ann Emerg
Med 21:1243-1245, 1991.
187. Neves MC, Brito U, Miguel MJP, Vivente O, Barros F, Silva
JR: Evaluation of Mg2+ aspartate HCl in asthmatic crises.
Magnesium Bull 13:88-93, 1991. 188. Okayama H, Okayama M, Aikawa
T, Sasaki M, Takishima T: Treatment of status asthmaticus with
intravenous magnesium sulfate. J Asthma 28:11-17, 1991.
189. Hauser SP, Braun PH: Intravenous magnesium in asthmatic
patients. A clinical trial and review of the literature. in
MAGNESIUM - A RELEVANT ION. Eds B Lasserre & J Durlach, Publ
John Libbey, London, Engl, pp 435-190. Manzke H, Thiemeier M,
Elster P, Lemke J: [Magnesium sulfate as adjuvant in
beta-2-sympathicomimetic inhalation therapy of bronchial asthma.]
Pneumologie 44:1190-1192, 1990. LA:GERMAN; EnglAbstr
191. Kufs WM (letter): Intravenous magnesium sulfate in acute
asthma. JAMA 263:516-517, 1990.
192. Green SM, Rothrock SG: Intravenous magnesium for acute
asthma: failure to decrease emergency treatment duration or need
for hospitalization. Ann Emerg Med 21:260-265, 1992.
193. Tiffany BR, Berk WA, Todd IK, White SR: Magnesium bolus
or infusion fails to improve expiratory flow in acute asthma
exacerbations. Chest 104:831-834, 1993.
194. Skobeloff EM, McNamara RM (letter): Intravenous magnesium
for acute asthma. Ann Emerg Med 22:617-618, 1993.
195. Spivey WH (letter): Intravenous magnesium for acute
asthma. Ann Emerg Med 22:617, 1993.
196. Fesmire FM (letter): Intravenous magnesium for acute
asthma. Ann Emerg Med 22:616-617, 1993.
197. Classen HG, Scherb H, Scherb I: [In-vitro studies on the
broncholytic efficacy of magnesium.] Magnesium Bull 11:80-84,
1989. (in German)
198. Spivey WH, Skobeloff EM, Levin RM: Effect of magnesium
chloride on rabbit bronchial smooth muscle. Ann Emerg Med
19:1107-1112, 1990.
199. Lindeman KS, Hirshman CA, Freed: Effect of magnesium
sulfate on bronchoconstriction in the lung periphery. J Appl
Physiol 66:2527-2532, 1989.
200. Mathew R, Altura BM: The role of magnesium in lung
diseases: asthma, allergy and pulmonary hypertension. Magnes
Trace Elem 10:220-228, 1991-92
201. Landon RA, Young EA:Role of magnesium in regulation of
lung function. J Am Dietet Assoc 93:674-677, 1993.
202. Keen CL, Lowney P, Gershwin ME, Hurley LS, Stern JS:
Dietary magnesium intake influences exercise capacity and
hematologic parameters in rats. Metabolism 36:788-793, 1987.
203. Lowney P, Gershwin ME, Stern JS, Keen CL: Magnesium
intake and endurance capacity in rats. Fed Proc 46:1020,
1987.
204. Lowney P, Gershwin ME, Hurley LS, Stern JS, Keen CL: The
effect of variable magnesium intake on potential factors
influencing endurance capacity. Biol Trace Elem Res 16:1-18,
1988.
205. Gershwin ME, Lowney P, Hurley LS, Keen CL: Effect of
variable magnesium intake on hematological and immunological
parameters in the rat. Abstr of presentation at Intl Sympos on
Mg, Arlington, VA, Sept, 1986. Magnesium 6:153, 1987.
206. Lowney P, Stern JS, Gershwin ME, Keen CL: Magnesium
deficiency and blood 2,3-diphosphoglycerate concentrations in
sedentary and exercised male Osborne-Mendel rats. Metabolism
39:837-841, 1990.
207. Laires MJ, Rayssiguier Y, Guezennec CY, Alves F, Halpern
MJ: Effect of magnesium deficiency on exercise capacity in rats.
Magnesium Res 2:136, 1989.
208. Hirneth HD, Classen HG: Swimming performance of rats in
relation to magnesium intake. Magnesium Res 1:98-99, 1988.
209. Refsum HE, Meen HD, Stromme S: Whole blood, serum and
erythrocyte magnesium concentrations after repeated heavy
exercise of long duration. Scand J Clin Lab Invest 32:123-127,
1973.
210. Casoni I, Guglielmini C, Graziano L, Reali MG, Mazzotta
D, Abbasciano V: Changes of magnesium concentrations in endurance
athletes. Intl J Sports Med 11:234-237, 1990.
211. Hoffmann G, Boehmer D: [Magnesium concentrations in whole
blood, serum and erythrocytes in trained long distance runners
before and after 25 kilometer race with and without four weeks
oral electrolyte intake.] Magnesium Bull 10:56-63, 1988. (in
German)
212. Lijnen P, Hespel P, Fagard R, Lysens R, Vanden-Eynde E,
Amery A: Erythrocyte, plasma and urinary magnesium in men before
and after a marathon. Europ J Appl Physiol 58:252-256, 1988.
213. Mader A, Hartmann U, Fischer HG, Reinhards-Mader G,
Bohnert KJ, Hollmann W: [The substitution of magnesium during
towing team's altitude training in the offing of the Olympic
games--results of a controlled study.] Magnesium Bull 12:69-78,
1990. (in German)
214. Jooste PL, Wolfswinkel JM, Schoeman JJ, Strydom NB:
Epileptic-Type Convulsions and Magnesium Deficiency. Aviation,
Space Environm Med 50:732-745, 1979.
215. Bertschat F, Golf S, Riediger H, Graef V, Ising H:
Protective effects of magnesium on release of proteins from
muscle cells during a marathon run. Magnesium Bull 8:310-313,
1986.
216. Stendig-Lindberg G, Epstein Y, Galun E, Shapiro Y, Graff
E, Schonberger E: Persistent hypomagnesaemia following strenuous
effort. Abstr Magnesium 4:212-213, 1985.
217. Stendig-Lindberg G, Shapiro Y, Epstein Y, Galun E,
Schonberger E, Graff E, Wacker WEC: Changes in serum magnesium
concentration after strenuous exercise. J Am Coll Nutr 6:35-40,
1987.
218. Stendig-Lindberg G, Shapiro Y, Graff E, Schonberger E,
Wacker WEC: Delayed metabolic changes after strenuous exertion in
trained young men. Magnesium Res 2:211-218, 1989.
219. Stendig-Lindberg G, Wacker WE, Shapiro Y: Long term
effects of peak strenuous effort on serum magnesium, lipids, and
blood sugar in apparently healthy young men. Magnes Res 4:59-65,
1991.
220. Boehmer D: [Reaction on magnesium serum level of athletic
performance.] Magnesium Bull 1:109-110, 1979. (in German)
221. Ansquer D: The influence of sports activity on magnesium
metabolism. Ph D Thesis, abstr in Magnesium Res. 1:122, 1988.
222. Deuster PA, Dolev E, Kyle SB, Anderson RA, Schoomaker EB:
Magnesium homeostasis during high-intensity anaerobic exercise in
men. J Appl Physiol 62:545-550, 1987.
223. Lukaski HC, Bolonchuk WW, Klevay LM, Milne DB, Sandstead
HH: Maximal oxygen consumption as related to magnesium, copper,
and zinc nutriture. Am J Clin Nutr 37:407-415, 1983.
224. Conn CA, Schemmel RA, Smith BW, Ryder E, Heusner WW, Ku
PK: Plasma and erythrocyte magnesium concentrations and
correlations with maximum oxygen consumption in nine- to
twelve-year-old competitive swimmers. Magnesium 7:27-36,
1988.
225. Laires MJ, Alves F, Halpern MJ: Changes in serum and
erythrocyte magnesium and blood lipids after distance swimming.
Magnesium Res 1:219-222, 1988.
226. Ahlborg B, Ekelund LG, Nilsson CG: Effect of
potassium-magnesium-aspartate on the capacity for prolonged
exercise in man. Acta Physiol Scand 74:238-245, 1968.
227. Bergstrom J, Guarnieri G, Hultman E: Carbohydrate
metabolism and electrolyte changes in human muscle tissue during
heavy work. J Appl Physiol 30:122-125, 1971.
228. Boehmer D: [Influence of endurance training on sodium,
potassium and magnesium homeostasis in swimmers: girls and boys].
Magnesium Bull 1:74-76, 1979. (in German)
229. deHaan A, vanDoorn JE, Westra HG: Effects of potassium +
magnesium aspartate on muscle metabolism and force development
during short intensive static exercise. Intl J Sports Med
6:44-49, 1985.
230. Veith J, Beuker F, Spannaus W: Magnesium-substitution in
runners and endurance athletes. Magnesium Res 1:99, 1988.
231. Terblanche S, Noakes TD, Dennis SC, Marais D, Eckert M:
Failure of magnesium supplementation to influence marathon
running performance or recovery in magnesium-replete subjects.
Intl J Sport Nutr 2:154-164, 1992.
232. Wodick R, Grunert-Fuchs M: [The influence of long term
magnesium administration in different parameters of physical
performance.] Magnesium Bull 7:51-55, 1985.
233. Golf S, Munch J, Graef V, Temme H, Brustle A, Roka L,
Beuther G, Heinz N, Buhl C, Nowacki PE: [Influence of magnesium
supplementation over 4 weeks on lactate elimination of rowers
during exhausting competition-specific performance.] Magnesium
Bull 10:124-130, 1988. (in German)
234. Pohlmann U, Schmidt M, Golf S, Graef V, Roka L, Temme H,
Riediger H, Neppel H, Baumgaertner Bortz C: [Effects of magnesium
on hemostasis in competitive swimmers before and after maximal
exercise.] Magnesium Bull 12:47-51, 1990. (in German)
235. Food and Nutrition Board, Natl Res Council: RECOMMENDED
DIETARY ALLOWANCES, 9th Ed. Washington, D.C., Natl Acad Press. pp
134-136, 1980.
236. Moffatt RJ: Dietary status of elite female high school
gymnasts: inadequacy of vitamin and mineral intake. J Am Diet
Assoc 84:1361-1363, 1984.
237. Deuster PA, Kyle SB, Moser PB, Vigersky RA, Singh A,
Schoomaker EB: Nutritional survey of highly trained women
runners. Am J Clin Nutr 44:954-962, 1986.
238. Boggio V, Guilland JC, Moreau D, Durlach J, Klepping J:
Dietary magnesium intake among male athletes in France. Magnesium
Bull 8:275, 1986.
239. Singh A, Deuster PA, Day BA, DeBolt JE, Doubt JT. Mineral
and electrolyte status of U.S. Navy divers. Fed Proc 46:592,
1987.
240. Keith R.E., O'Keeffe K.A., Alt L.A., Young K.L.(1989):
Dietary status of trained female cyclists. J Am Diet Assoc
89:1620-1623.
241. Singh A, Day BA, DeBolt JE, Trostmann UH, Bernier LL,
Deuster PA: Magnesium, zinc, and copper status of US Navy SEAL
trainees. Am J Clin Nutr 49:695-700, 1989.
242. Singh A, Deuster PA, Day BA, Moser-Veillon PB: Dietary
intakes and biochemical markers of selected minerals: comparison
of highly trained runners and untrained women. J Am Coll Nutr 9
65-75, 1990.
243. Durlach J: Recommended dietary amounts of magnesium: Mg
RDA. Magnesium Res 2:195-203, 1989.
245. Seelig MS: Adverse stress reactions in magnesium
deficiency; preventive and therapeutic implications. abstr of
poster presentation, 33rd Annual Sympos of ACN, J Am Coll Nutr
11:609, 1992.
246. Rayssiguier Y, Guezennec CY, Durlach J: New experimental
and clinical data on the relationship between magnesium and
sport. Magnes Res 3: 93-102, 1990.
247. Niemack EA, Stoeckli F, Husmann E, Sonderegger J, Classen
HG, Helbig J: [The effect of magnesium aspartate hydrochloride on
cannibalism, transport stress and electrolyte content in the
heart of pigs.] Magnesium Bull 1:195-198, 1979. (in German)
248. Schumm H: [The effect of
magnesium-1-aspartate-hydrochloride on transport losses with
fattening pigs and piglets as well as on the meat quality of
carcasses.] Magnesium Bull 6:23-29, 1984. (in German)
249. Niemack EA: [Magnesium. Mineral--trace
element--therapeutic substance? A review.] Schweiz Arch
Tierheilkd 127:597-604, 1985.(in German)
250. Grashorn M, Schimatschek HF, Hickl R, Scholtyssek S,
Classen HG: [The influence of
monomagnesium-L-aspartate-hydrochloride on the "sudden death
syndrome" and electrolyte distribution in broilers.] Magnesium
Bull 10:9-14, 1988. (in German)
251. Rasmussen HS: Justification for intravenous magnesium
therapy in acute myocardial infarction. Magnesium Res 1:59-73,
1988.
252. Rasmussen HS: Clinical intervention studies on magnesium
in myocardial infarction. Magnesium 8:316-325, 1989.
253. Shechter M: Beneficial effects of magnesium in acute
myocardial infarction. A review of the literature. Magnesium Bull
12:1-6, 1990.
254. Woods KL, Fletcher S, Roffe C, Haider Y: Intravenous
magnesium sulphate in suspected acute myocardial infarction:
results of the second Leicester Intravenous Magnesium
Intervention Trial (LIMIT-2). Lancet 339:1553-1558, 1992.
255. Woods KL, Fletcher S: Long-term outcome after intravenous
magnesium sulphate in suspected acute myocardial infarction: the
second Leicester Intravenous Magnesium Intervention Trial
(LIMIT-2). Lancet 343:816-819, 1994.
256. Casscells W: Magnesium and myocardial infarction. Lancet
343:807-809, 1994.
257. NIH Consensus Development Conference on Optimal Calcium
Intake. Bethesda, MD, June 6-8, 1994.
258. Karppannen H, Pennanen R, Passinen L: Minerals, coronary
heart disease and sudden coronary death. Adv Cardiol 25:9-24,
1978.
259. Karppannen H: Epidemiologic evidence for considering
magnesium deficiency as a risk factor for cardiovascular
diseases. Mg Bull 12:80-86, 1990.
(This article has been placed on this web site with the
permission of the Journal of the American College of Nutrition.
We thank Dr. Mildred S. Seelig for providing us the information
on a diskette.)
This page was first uploaded to The Magnesium Web Site on
October 18, 1995
http://www.mgwater.com/



